 Found 9 hits for monomerid = 50309888
Found 9 hits for monomerid = 50309888 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor
(Rattus norvegicus (Rat)) | BDBM50309888
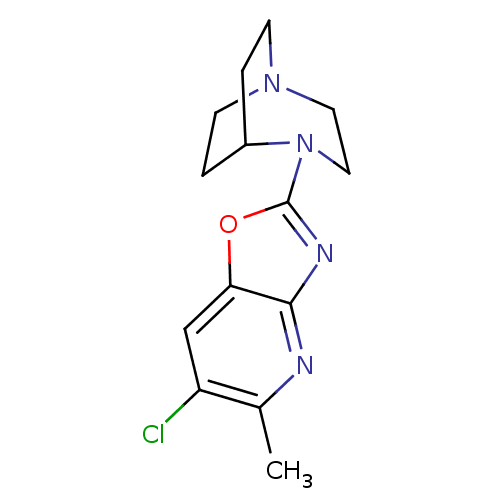
(4-(6-Chloro-5-methyloxazolo[4,5-b]pyridin-2-yl)-1,...)Show SMILES Cc1nc2nc(oc2cc1Cl)N1CCN2CCC1CC2 |TLB:5:11:16.15:18.19,(1.04,-10.22,;-.49,-10.46,;-1.05,-11.9,;-2.58,-12.13,;-3.43,-13.41,;-4.92,-13,;-4.98,-11.47,;-3.54,-10.92,;-2.99,-9.49,;-1.46,-9.25,;-.9,-7.79,;-6.25,-13.78,;-5.89,-15.24,;-6.89,-14.52,;-8.27,-14.59,;-8.34,-12.89,;-7.73,-11.74,;-7.66,-13.15,;-8.96,-13.86,;-9.26,-15.3,)| Show InChI InChI=1S/C14H17ClN4O/c1-9-11(15)8-12-13(16-9)17-14(20-12)19-7-6-18-4-2-10(19)3-5-18/h8,10H,2-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor protein alpha-7 subunit
(Homo sapiens (Human)) | BDBM50309888

(4-(6-Chloro-5-methyloxazolo[4,5-b]pyridin-2-yl)-1,...)Show SMILES Cc1nc2nc(oc2cc1Cl)N1CCN2CCC1CC2 |TLB:5:11:16.15:18.19,(1.04,-10.22,;-.49,-10.46,;-1.05,-11.9,;-2.58,-12.13,;-3.43,-13.41,;-4.92,-13,;-4.98,-11.47,;-3.54,-10.92,;-2.99,-9.49,;-1.46,-9.25,;-.9,-7.79,;-6.25,-13.78,;-5.89,-15.24,;-6.89,-14.52,;-8.27,-14.59,;-8.34,-12.89,;-7.73,-11.74,;-7.66,-13.15,;-8.96,-13.86,;-9.26,-15.3,)| Show InChI InChI=1S/C14H17ClN4O/c1-9-11(15)8-12-13(16-9)17-14(20-12)19-7-6-18-4-2-10(19)3-5-18/h8,10H,2-7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to alpha7 nAChR |
J Med Chem 54: 7943-61 (2011)
Article DOI: 10.1021/jm2007672
BindingDB Entry DOI: 10.7270/Q29P32QP |
More data for this
Ligand-Target Pair | |
5-HT3A Serotonin Receptor
(Mus musculus (house mouse)) | BDBM50309888

(4-(6-Chloro-5-methyloxazolo[4,5-b]pyridin-2-yl)-1,...)Show SMILES Cc1nc2nc(oc2cc1Cl)N1CCN2CCC1CC2 |TLB:5:11:16.15:18.19,(1.04,-10.22,;-.49,-10.46,;-1.05,-11.9,;-2.58,-12.13,;-3.43,-13.41,;-4.92,-13,;-4.98,-11.47,;-3.54,-10.92,;-2.99,-9.49,;-1.46,-9.25,;-.9,-7.79,;-6.25,-13.78,;-5.89,-15.24,;-6.89,-14.52,;-8.27,-14.59,;-8.34,-12.89,;-7.73,-11.74,;-7.66,-13.15,;-8.96,-13.86,;-9.26,-15.3,)| Show InChI InChI=1S/C14H17ClN4O/c1-9-11(15)8-12-13(16-9)17-14(20-12)19-7-6-18-4-2-10(19)3-5-18/h8,10H,2-7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]LY278584 from mouse 5HT3 receptor expressed in HEK293 cells |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50309888

(4-(6-Chloro-5-methyloxazolo[4,5-b]pyridin-2-yl)-1,...)Show SMILES Cc1nc2nc(oc2cc1Cl)N1CCN2CCC1CC2 |TLB:5:11:16.15:18.19,(1.04,-10.22,;-.49,-10.46,;-1.05,-11.9,;-2.58,-12.13,;-3.43,-13.41,;-4.92,-13,;-4.98,-11.47,;-3.54,-10.92,;-2.99,-9.49,;-1.46,-9.25,;-.9,-7.79,;-6.25,-13.78,;-5.89,-15.24,;-6.89,-14.52,;-8.27,-14.59,;-8.34,-12.89,;-7.73,-11.74,;-7.66,-13.15,;-8.96,-13.86,;-9.26,-15.3,)| Show InChI InChI=1S/C14H17ClN4O/c1-9-11(15)8-12-13(16-9)17-14(20-12)19-7-6-18-4-2-10(19)3-5-18/h8,10H,2-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT3 receptor |
J Med Chem 54: 7943-61 (2011)
Article DOI: 10.1021/jm2007672
BindingDB Entry DOI: 10.7270/Q29P32QP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50309888

(4-(6-Chloro-5-methyloxazolo[4,5-b]pyridin-2-yl)-1,...)Show SMILES Cc1nc2nc(oc2cc1Cl)N1CCN2CCC1CC2 |TLB:5:11:16.15:18.19,(1.04,-10.22,;-.49,-10.46,;-1.05,-11.9,;-2.58,-12.13,;-3.43,-13.41,;-4.92,-13,;-4.98,-11.47,;-3.54,-10.92,;-2.99,-9.49,;-1.46,-9.25,;-.9,-7.79,;-6.25,-13.78,;-5.89,-15.24,;-6.89,-14.52,;-8.27,-14.59,;-8.34,-12.89,;-7.73,-11.74,;-7.66,-13.15,;-8.96,-13.86,;-9.26,-15.3,)| Show InChI InChI=1S/C14H17ClN4O/c1-9-11(15)8-12-13(16-9)17-14(20-12)19-7-6-18-4-2-10(19)3-5-18/h8,10H,2-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at human 5HT3 receptor expressed in human skin epithelial cells assessed as stimulation of calcium flux by FLIPR assay |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50309888

(4-(6-Chloro-5-methyloxazolo[4,5-b]pyridin-2-yl)-1,...)Show SMILES Cc1nc2nc(oc2cc1Cl)N1CCN2CCC1CC2 |TLB:5:11:16.15:18.19,(1.04,-10.22,;-.49,-10.46,;-1.05,-11.9,;-2.58,-12.13,;-3.43,-13.41,;-4.92,-13,;-4.98,-11.47,;-3.54,-10.92,;-2.99,-9.49,;-1.46,-9.25,;-.9,-7.79,;-6.25,-13.78,;-5.89,-15.24,;-6.89,-14.52,;-8.27,-14.59,;-8.34,-12.89,;-7.73,-11.74,;-7.66,-13.15,;-8.96,-13.86,;-9.26,-15.3,)| Show InChI InChI=1S/C14H17ClN4O/c1-9-11(15)8-12-13(16-9)17-14(20-12)19-7-6-18-4-2-10(19)3-5-18/h8,10H,2-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 53: 1222-37 (2010)
Article DOI: 10.1021/jm9015075
BindingDB Entry DOI: 10.7270/Q2QN66W3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50309888

(4-(6-Chloro-5-methyloxazolo[4,5-b]pyridin-2-yl)-1,...)Show SMILES Cc1nc2nc(oc2cc1Cl)N1CCN2CCC1CC2 |TLB:5:11:16.15:18.19,(1.04,-10.22,;-.49,-10.46,;-1.05,-11.9,;-2.58,-12.13,;-3.43,-13.41,;-4.92,-13,;-4.98,-11.47,;-3.54,-10.92,;-2.99,-9.49,;-1.46,-9.25,;-.9,-7.79,;-6.25,-13.78,;-5.89,-15.24,;-6.89,-14.52,;-8.27,-14.59,;-8.34,-12.89,;-7.73,-11.74,;-7.66,-13.15,;-8.96,-13.86,;-9.26,-15.3,)| Show InChI InChI=1S/C14H17ClN4O/c1-9-11(15)8-12-13(16-9)17-14(20-12)19-7-6-18-4-2-10(19)3-5-18/h8,10H,2-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc
Curated by ChEMBL
| Assay Description
Inhibition of 5HT3 receptor |
J Med Chem 54: 7943-61 (2011)
Article DOI: 10.1021/jm2007672
BindingDB Entry DOI: 10.7270/Q29P32QP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor protein alpha-7 subunit
(Homo sapiens (Human)) | BDBM50309888

(4-(6-Chloro-5-methyloxazolo[4,5-b]pyridin-2-yl)-1,...)Show SMILES Cc1nc2nc(oc2cc1Cl)N1CCN2CCC1CC2 |TLB:5:11:16.15:18.19,(1.04,-10.22,;-.49,-10.46,;-1.05,-11.9,;-2.58,-12.13,;-3.43,-13.41,;-4.92,-13,;-4.98,-11.47,;-3.54,-10.92,;-2.99,-9.49,;-1.46,-9.25,;-.9,-7.79,;-6.25,-13.78,;-5.89,-15.24,;-6.89,-14.52,;-8.27,-14.59,;-8.34,-12.89,;-7.73,-11.74,;-7.66,-13.15,;-8.96,-13.86,;-9.26,-15.3,)| Show InChI InChI=1S/C14H17ClN4O/c1-9-11(15)8-12-13(16-9)17-14(20-12)19-7-6-18-4-2-10(19)3-5-18/h8,10H,2-7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
Targacept, Inc
Curated by ChEMBL
| Assay Description
Agonist activity at alpha7 nAChR |
J Med Chem 54: 7943-61 (2011)
Article DOI: 10.1021/jm2007672
BindingDB Entry DOI: 10.7270/Q29P32QP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50309888

(4-(6-Chloro-5-methyloxazolo[4,5-b]pyridin-2-yl)-1,...)Show SMILES Cc1nc2nc(oc2cc1Cl)N1CCN2CCC1CC2 |TLB:5:11:16.15:18.19,(1.04,-10.22,;-.49,-10.46,;-1.05,-11.9,;-2.58,-12.13,;-3.43,-13.41,;-4.92,-13,;-4.98,-11.47,;-3.54,-10.92,;-2.99,-9.49,;-1.46,-9.25,;-.9,-7.79,;-6.25,-13.78,;-5.89,-15.24,;-6.89,-14.52,;-8.27,-14.59,;-8.34,-12.89,;-7.73,-11.74,;-7.66,-13.15,;-8.96,-13.86,;-9.26,-15.3,)| Show InChI InChI=1S/C14H17ClN4O/c1-9-11(15)8-12-13(16-9)17-14(20-12)19-7-6-18-4-2-10(19)3-5-18/h8,10H,2-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 54: 7943-61 (2011)
Article DOI: 10.1021/jm2007672
BindingDB Entry DOI: 10.7270/Q29P32QP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data