

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50309889 2-(5-Ethyloxazolo[4,5-b]pyridin-2-yl)-2,5-diazabicyclo[3.2.2]-nonane::CHEMBL598555
SMILES: CCc1ccc2oc(nc2n1)N1CCN2CCC1CC2
InChI Key: InChIKey=YABLRALLVGHPFP-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor (Rattus norvegicus (Rat)) | BDBM50309889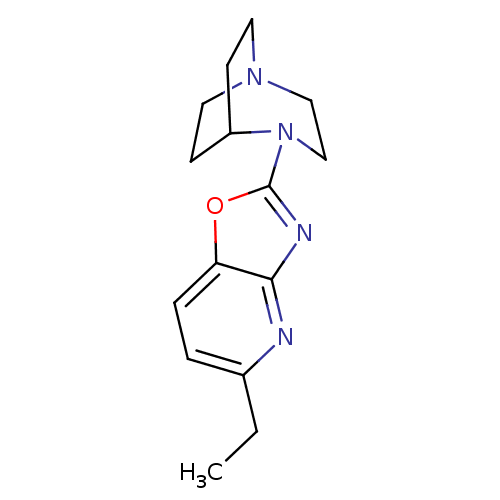 (2-(5-Ethyloxazolo[4,5-b]pyridin-2-yl)-2,5-diazabic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-HT3A Serotonin Receptor (Mus musculus (house mouse)) | BDBM50309889 (2-(5-Ethyloxazolo[4,5-b]pyridin-2-yl)-2,5-diazabic...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]LY278584 from mouse 5HT3 receptor expressed in HEK293 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50309889 (2-(5-Ethyloxazolo[4,5-b]pyridin-2-yl)-2,5-diazabic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity at human 5HT3 receptor expressed in human skin epithelial cells assessed as stimulation of calcium flux by FLIPR assay | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50309889 (2-(5-Ethyloxazolo[4,5-b]pyridin-2-yl)-2,5-diazabic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||