

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50310381 (S)-5-[4-(2-Piperidin-1-yl-ethoxy)-phenyl]-11,12-dihydro-5H-6,13-dioxa-benzo[3,4]cyclohepta[1,2-a]naphthalene-2,8-diol::CHEMBL1088631
SMILES: Oc1ccc2C3=C([C@@H](Oc2c1)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1OCC3
InChI Key: InChIKey=OFYZVZJXVRFMIW-PMERELPUSA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol receptor beta (ERβ) (Homo sapiens (Human)) | BDBM50310381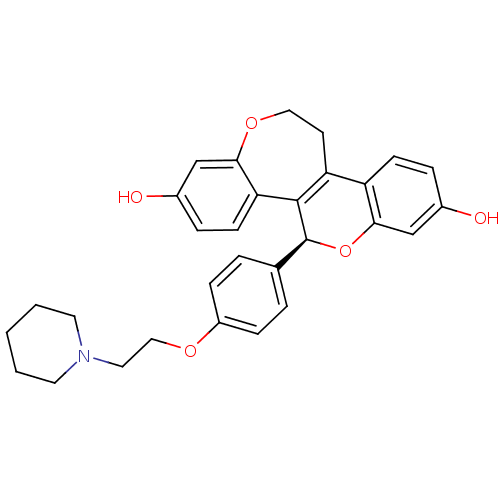 ((S)-5-[4-(2-Piperidin-1-yl-ethoxy)-phenyl]-11,12-d...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of fluormone ES2 binding to estrogen receptor beta after 1 hr by fluorescence polarization assay | J Med Chem 52: 7544-69 (2009) Article DOI: 10.1021/jm900146e BindingDB Entry DOI: 10.7270/Q27D2V8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50310381 ((S)-5-[4-(2-Piperidin-1-yl-ethoxy)-phenyl]-11,12-d...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC Curated by ChEMBL | Assay Description Displacement of radiolabeled estrogen from estrogen receptor alpha by scintillation counting | J Med Chem 52: 7544-69 (2009) Article DOI: 10.1021/jm900146e BindingDB Entry DOI: 10.7270/Q27D2V8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50310381 ((S)-5-[4-(2-Piperidin-1-yl-ethoxy)-phenyl]-11,12-d...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC Curated by ChEMBL | Assay Description Displacement of radiolabeled estrogen from estrogen receptor alpha by scintillation counting | J Med Chem 52: 7544-69 (2009) Article DOI: 10.1021/jm900146e BindingDB Entry DOI: 10.7270/Q27D2V8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50310381 ((S)-5-[4-(2-Piperidin-1-yl-ethoxy)-phenyl]-11,12-d...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor in human MCF7 cells assessed as 17beta-estradiol-induced cell proliferation after 24 hrs by [14C]thymidine i... | J Med Chem 52: 7544-69 (2009) Article DOI: 10.1021/jm900146e BindingDB Entry DOI: 10.7270/Q27D2V8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50310381 ((S)-5-[4-(2-Piperidin-1-yl-ethoxy)-phenyl]-11,12-d...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor in human Ishikawa cells assessed as 17beta-estradiol-induced alkaline phosphatase activity after 3 days by c... | J Med Chem 52: 7544-69 (2009) Article DOI: 10.1021/jm900146e BindingDB Entry DOI: 10.7270/Q27D2V8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50310381 ((S)-5-[4-(2-Piperidin-1-yl-ethoxy)-phenyl]-11,12-d...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor in human MCF7 cells assessed as 17beta-estradiol-induced cell proliferation after 24 hrs by [14C]thymidine i... | J Med Chem 52: 7544-69 (2009) Article DOI: 10.1021/jm900146e BindingDB Entry DOI: 10.7270/Q27D2V8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50310381 ((S)-5-[4-(2-Piperidin-1-yl-ethoxy)-phenyl]-11,12-d...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor in human Ishikawa cells assessed as 17beta-estradiol-induced alkaline phosphatase activity after 3 days by c... | J Med Chem 52: 7544-69 (2009) Article DOI: 10.1021/jm900146e BindingDB Entry DOI: 10.7270/Q27D2V8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estradiol receptor beta (ERβ) (Homo sapiens (Human)) | BDBM50310381 ((S)-5-[4-(2-Piperidin-1-yl-ethoxy)-phenyl]-11,12-d...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of fluormone ES2 binding to estrogen receptor beta after 1 hr by fluorescence polarization assay | J Med Chem 52: 7544-69 (2009) Article DOI: 10.1021/jm900146e BindingDB Entry DOI: 10.7270/Q27D2V8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||