

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Oc1ccc2C3=C(CCOc2c1)c1ccc(F)cc1O[C@@H]3c1ccc(OCCN2CCCCCC2)cc1
InChI Key: InChIKey=KLRGEDJTDSUZSF-WJOKGBTCSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50310404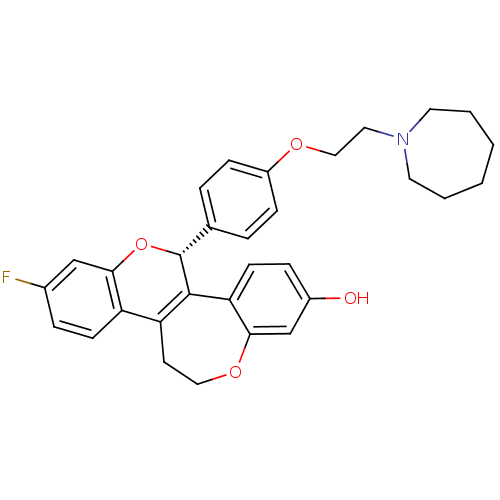 ((R)-5-[4-(2-Azepan-1-yl-ethoxy)-phenyl]-8-fluoro-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of fluormone ES2 binding to estrogen receptor beta after 1 hr by fluorescence polarization assay | J Med Chem 52: 7544-69 (2009) Article DOI: 10.1021/jm900146e BindingDB Entry DOI: 10.7270/Q27D2V8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50310404 ((R)-5-[4-(2-Azepan-1-yl-ethoxy)-phenyl]-8-fluoro-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor in human MCF7 cells assessed as 17beta-estradiol-induced cell proliferation after 24 hrs by [14C]thymidine i... | J Med Chem 52: 7544-69 (2009) Article DOI: 10.1021/jm900146e BindingDB Entry DOI: 10.7270/Q27D2V8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50310404 ((R)-5-[4-(2-Azepan-1-yl-ethoxy)-phenyl]-8-fluoro-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor in human Ishikawa cells assessed as 17beta-estradiol-induced alkaline phosphatase activity after 3 days by c... | J Med Chem 52: 7544-69 (2009) Article DOI: 10.1021/jm900146e BindingDB Entry DOI: 10.7270/Q27D2V8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50310404 ((R)-5-[4-(2-Azepan-1-yl-ethoxy)-phenyl]-8-fluoro-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC Curated by ChEMBL | Assay Description Displacement of radiolabeled estrogen from estrogen receptor alpha by scintillation counting | J Med Chem 52: 7544-69 (2009) Article DOI: 10.1021/jm900146e BindingDB Entry DOI: 10.7270/Q27D2V8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||