

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50310540 ((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl dihydrogen phosphate::((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl dihydrogen phosphate::CHEMBL307679::CYTIDINE-5'-MONOPHOSPHATE::Phosphoric acid mono-[5-(4-amino-2-oxo-2H-pyrimidin-1-yl)-3,4-dihydroxy-tetrahydro-furan-2-ylmethyl] ester::cytidine 5'-phosphate
SMILES: Nc1ccn([C@@H]2O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]2O)c(=O)n1
InChI Key: InChIKey=IERHLVCPSMICTF-XVFCMESISA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CMP-N-acetylneuraminate-beta-galactosamide-alpha-2,6-sialyltransferase (Rattus norvegicus) | BDBM50310540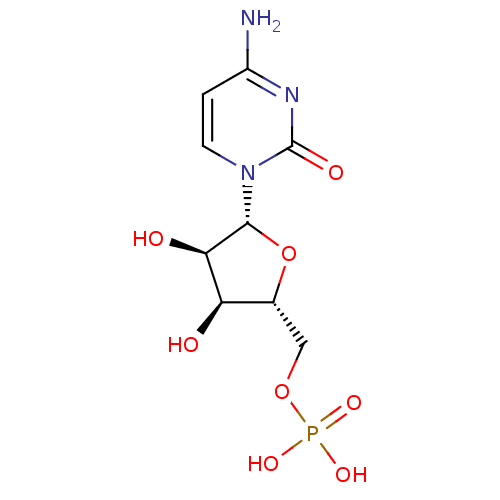 (((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase (Rattus norvegicus) | BDBM50310540 (((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,3-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CAM-RNase A (Bison bison (American bison)) | BDBM50310540 (((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
National Hellenic Research Foundation Curated by ChEMBL | Assay Description Inhibition of bovine pancreatic ribonuclease A assessed as enzyme activity by spectrophotometric method pH 6 | Eur J Med Chem 44: 4496-508 (2009) Article DOI: 10.1016/j.ejmech.2009.06.039 BindingDB Entry DOI: 10.7270/Q26H4HJJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Orotidine Monophosphate Decarboxylase (ODCase) (Methanobacterium thermoautotrophicum) | BDBM50310540 (((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of Methanobacterium thermoautotrophicum ODCase by isothermal titration calorimetry | J Med Chem 55: 9988-97 (2012) Article DOI: 10.1021/jm301176r BindingDB Entry DOI: 10.7270/Q2DR2WPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (HsOPRT) (Homo sapiens (Human)) | BDBM50310540 (((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of human ODCase by isothermal titration calorimetry | J Med Chem 55: 9988-97 (2012) Article DOI: 10.1021/jm301176r BindingDB Entry DOI: 10.7270/Q2DR2WPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Seminal ribonuclease (Bos taurus) | BDBM50310540 (((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.56E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
National Hellenic Research Foundation Curated by ChEMBL | Assay Description Inhibition of bovine seminal ribonuclease assessed as enzyme activity by spectrophotometric method at pH 6 | Eur J Med Chem 44: 4496-508 (2009) Article DOI: 10.1016/j.ejmech.2009.06.039 BindingDB Entry DOI: 10.7270/Q26H4HJJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Orotidine Monophosphate Decarboxylase (ODCase) (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM50310540 (((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum ODCase by isothermal titration calorimetry | J Med Chem 55: 9988-97 (2012) Article DOI: 10.1021/jm301176r BindingDB Entry DOI: 10.7270/Q2DR2WPD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||