

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50311288 CHEMBL1078785::N-(2-chlorophenyl)pyrazolo[1,5-a]pyridine-3-carboxamide
SMILES: Clc1ccccc1NC(=O)c1cnn2ccccc12
InChI Key: InChIKey=CPQRFZBWMKEHSA-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ephrin type-B receptor 3 (EPHB3) (Homo sapiens (Human)) | BDBM50311288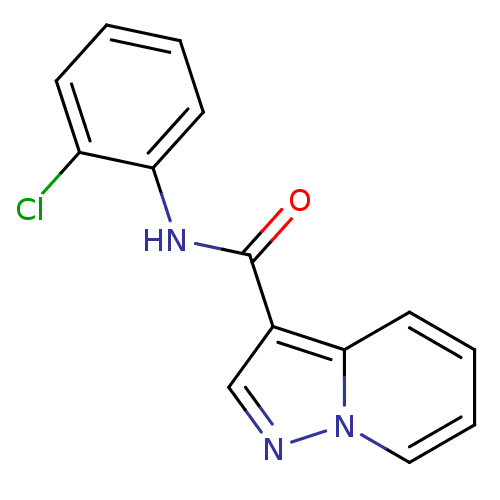 (CHEMBL1078785 | N-(2-chlorophenyl)pyrazolo[1,5-a]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50311288 (CHEMBL1078785 | N-(2-chlorophenyl)pyrazolo[1,5-a]p...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Lck (unknown origin) | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase RIPK2 (Homo sapiens (Human)) | BDBM50311288 (CHEMBL1078785 | N-(2-chlorophenyl)pyrazolo[1,5-a]p...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of full length RIPK2 (unknown origin) pretreated for 30 mins followed by ATP addition measured after 2 hrs by ADP-d2 tracer based fluoresc... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||