

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50315615 7-(3-(trifluoromethyl)pyridin-2-yl)-N-(5-(trifluoromethyl)pyridin-2-yl)-1,8-naphthyridin-4-amine::7-[3-(Trifluoromethyl)pyridin-2-yl]-N-[5-(trifluoromethyl)-pyridin-2-yl]-1,8-naphthyridin-4-amine::CHEMBL1089119
SMILES: FC(F)(F)c1ccc(Nc2ccnc3nc(ccc23)-c2ncccc2C(F)(F)F)nc1
InChI Key: InChIKey=ZDSWMSRFTNIEJV-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50315615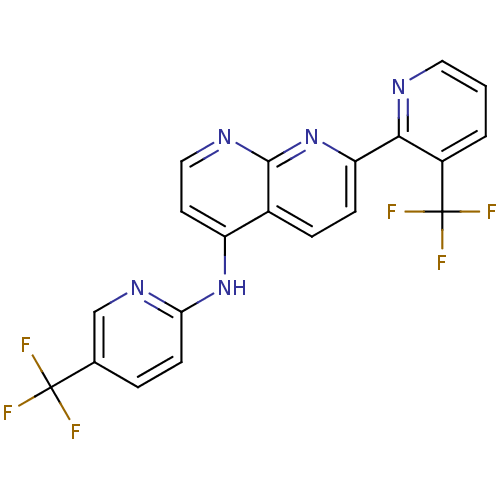 (7-(3-(trifluoromethyl)pyridin-2-yl)-N-(5-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced receptor activation by FLIPR assay | Bioorg Med Chem Lett 20: 4359-63 (2010) Article DOI: 10.1016/j.bmcl.2010.06.069 BindingDB Entry DOI: 10.7270/Q2959HRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vanilloid receptor (Rattus norvegicus (rat)) | BDBM50315615 (7-(3-(trifluoromethyl)pyridin-2-yl)-N-(5-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at Sprague-Dawley rat dorsal root ganglion TRPV1 assessed as inhibition of pH (5.0 to 5.5)-induced receptor activation | Bioorg Med Chem Lett 20: 4359-63 (2010) Article DOI: 10.1016/j.bmcl.2010.06.069 BindingDB Entry DOI: 10.7270/Q2959HRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 3 (Homo sapiens (Human)) | BDBM50315615 (7-(3-(trifluoromethyl)pyridin-2-yl)-N-(5-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Inhibition of human ERK1 | J Med Chem 53: 3330-48 (2010) Article DOI: 10.1021/jm100051g BindingDB Entry DOI: 10.7270/Q2474BTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vanilloid receptor (Rattus norvegicus (rat)) | BDBM50315615 (7-(3-(trifluoromethyl)pyridin-2-yl)-N-(5-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.25 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Inhibition of rat TRPV1 at pH 5 to 5.5 | J Med Chem 53: 3330-48 (2010) Article DOI: 10.1021/jm100051g BindingDB Entry DOI: 10.7270/Q2474BTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50315615 (7-(3-(trifluoromethyl)pyridin-2-yl)-N-(5-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ... | J Med Chem 53: 3330-48 (2010) Article DOI: 10.1021/jm100051g BindingDB Entry DOI: 10.7270/Q2474BTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||