

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50316818 ((2S,3aS,7aS)-1-((1R,2R)-2-phenylcyclopropanecarbonyl)octahydro-1H-indol-2-yl)(thiazolidin-3-yl)methanone::CHEMBL1086968
SMILES: O=C([C@@H]1C[C@H]1c1ccccc1)N1[C@H]2CCCC[C@H]2C[C@H]1C(=O)N1CCSC1
InChI Key: InChIKey=NXSXRIHXEQSYEZ-KNJMJIDISA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50316818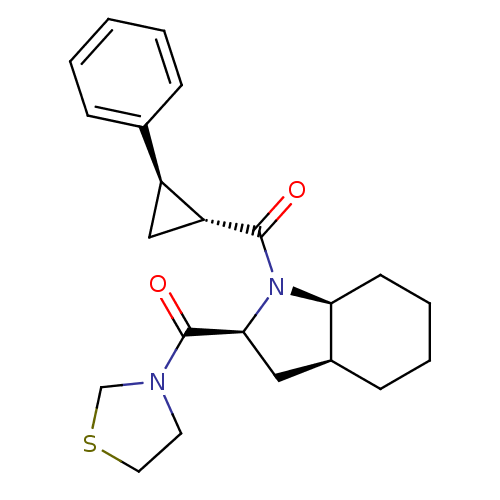 (((2S,3aS,7aS)-1-((1R,2R)-2-phenylcyclopropanecarbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description inhibition of rat cortex POP | J Med Chem 53: 3423-38 (2010) Article DOI: 10.1021/jm901104g BindingDB Entry DOI: 10.7270/Q261119N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50316818 (((2S,3aS,7aS)-1-((1R,2R)-2-phenylcyclopropanecarbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP using Z-Gly-Pro-7-AMC substrate | J Med Chem 59: 4221-34 (2016) BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50316818 (((2S,3aS,7aS)-1-((1R,2R)-2-phenylcyclopropanecarbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP | J Med Chem 53: 3423-38 (2010) Article DOI: 10.1021/jm901104g BindingDB Entry DOI: 10.7270/Q261119N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50316818 (((2S,3aS,7aS)-1-((1R,2R)-2-phenylcyclopropanecarbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant POP expressed in sEscherichia coli | Bioorg Med Chem Lett 18: 4360-3 (2008) Article DOI: 10.1016/j.bmcl.2008.06.067 BindingDB Entry DOI: 10.7270/Q20K2CCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||