

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50317147 2-(3-(-(S)-5-((S)-1-Amino-3-(3-chlorophenyl)-1-oxopropan-2-ylamino)-4-(3-hydroxy-4-methyl-2-nitrobenzamido)-5-oxopentylcarbamoyl)-phenoxy)acetic Acid::CHEMBL1094072
SMILES: Cc1ccc(C(=O)N[C@@H](CCCNC(=O)c2cccc(OCC(O)=O)c2)C(=O)N[C@@H](Cc2cccc(Cl)c2)C(N)=O)c(c1O)[N+]([O-])=O
InChI Key: InChIKey=DFNZCULYAYFVQZ-ZEQRLZLVSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317147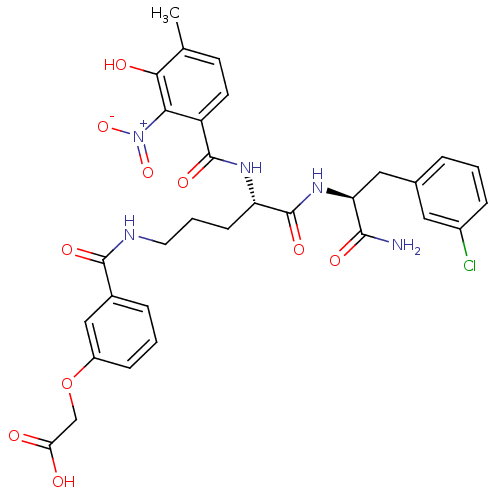 (2-(3-(-(S)-5-((S)-1-Amino-3-(3-chlorophenyl)-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase entC (Escherichia coli (strain K12)) | BDBM50317147 (2-(3-(-(S)-5-((S)-1-Amino-3-(3-chlorophenyl)-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminodeoxychorismate lyase (Escherichia coli (strain K12)) | BDBM50317147 (2-(3-(-(S)-5-((S)-1-Amino-3-(3-chlorophenyl)-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 ADCS by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||