

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50320748 CHEMBL1164243::N-((R)-5-((1R,3aS,7aR,E)-4-(2-((3R,5R)-3,5-Dihydroxycycloheptylidene)ethylidene)-7a-methyloctahydro-1H-inden-1-yl)hexyl)methanesulfamide
SMILES: [#6]-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]S([#7])(=O)=O)-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6]-[#6@H](-[#8])-[#6]-1
InChI Key: InChIKey=LOCGBKCIQGOTKO-YLPIWGMZSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50320748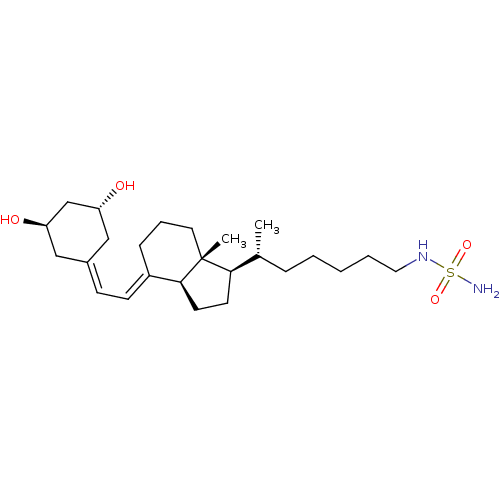 (CHEMBL1164243 | N-((R)-5-((1R,3aS,7aR,E)-4-(2-((3R...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HDAC3 after 10 mins by fluorometric assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50320748 (CHEMBL1164243 | N-((R)-5-((1R,3aS,7aR,E)-4-(2-((3R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR ligand binding domain by fluorescence polarization competition assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cereblon/Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50320748 (CHEMBL1164243 | N-((R)-5-((1R,3aS,7aR,E)-4-(2-((3R...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HDAC6 after 10 mins by fluorometric assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||