

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50322604 2-mercaptophenylphosphonic acid::CHEMBL1173336
SMILES: OP(O)(=O)c1ccccc1S
InChI Key: InChIKey=XUDMIKRWJYMQKD-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intestinal alkaline phosphatase (IAP) (Bos taurus (Cattle)) | BDBM50322604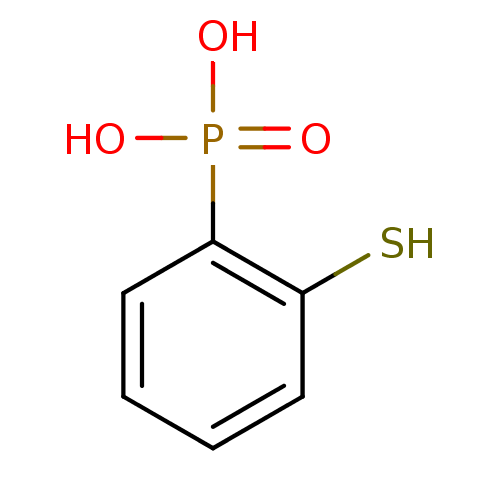 (2-mercaptophenylphosphonic acid | CHEMBL1173336) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase | J Med Chem 53: 4862-76 (2010) Article DOI: 10.1021/jm100213c BindingDB Entry DOI: 10.7270/Q2057G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Aeromonas hydrophila) | BDBM50322604 (2-mercaptophenylphosphonic acid | CHEMBL1173336) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge Curated by ChEMBL | Assay Description Inhibition of Aeromonas hydrophila cphA | J Med Chem 53: 4862-76 (2010) Article DOI: 10.1021/jm100213c BindingDB Entry DOI: 10.7270/Q2057G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase L1 (Stenotrophomonas maltophilia) | BDBM50322604 (2-mercaptophenylphosphonic acid | CHEMBL1173336) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge Curated by ChEMBL | Assay Description Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in absence of Zn+ chelator | J Med Chem 53: 4862-76 (2010) Article DOI: 10.1021/jm100213c BindingDB Entry DOI: 10.7270/Q2057G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase L1 (Stenotrophomonas maltophilia) | BDBM50322604 (2-mercaptophenylphosphonic acid | CHEMBL1173336) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge Curated by ChEMBL | Assay Description Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in presence of Zn+ chelator | J Med Chem 53: 4862-76 (2010) Article DOI: 10.1021/jm100213c BindingDB Entry DOI: 10.7270/Q2057G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fe(3+)-Zn(2+) purple acid phosphatase (Phaseolus vulgaris) | BDBM50322604 (2-mercaptophenylphosphonic acid | CHEMBL1173336) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of red kidney beans purple acid phosphatase | Eur J Med Chem 76: 132-44 (2014) Article DOI: 10.1016/j.ejmech.2014.02.008 BindingDB Entry DOI: 10.7270/Q2T43VM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||