

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50323707 CHEMBL1213160
SMILES: O[C@H]1[C@@H](OCCNC(=O)NCCO[C@@H]2[C@H](OP(O)(O)=O)[C@@H](OP(O)(O)=O)[C@H](OP(O)(O)O)[C@@H](OP(O)(O)=O)[C@@H]2OP(O)(O)=O)[C@@H](OP(O)(O)=O)[C@H](O)[C@@H](OP(O)(O)=O)[C@@H]1OP(O)(O)=O
InChI Key: InChIKey=UEFBKOOXHRKKMH-GUSBONCTSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323707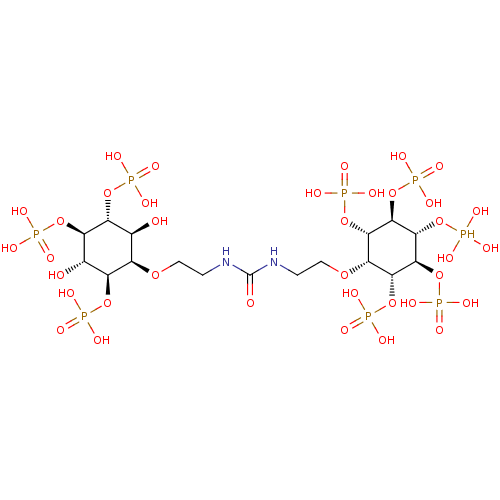 (CHEMBL1213160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Agonist activity at rat recombinant IP3R1 expressed in chicken DT40 cells assessed as calcium release from intracellular stores | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323707 (CHEMBL1213160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant IP3R1 binding core residue (224-604) expressed in chicken DT40 cells by equilibrium competitive binding ... | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323707 (CHEMBL1213160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant N-terminal IP3R1 (1-604) expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323707 (CHEMBL1213160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from full-length rat recombinant IP3R1 expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||