| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Inositol 1,4,5-trisphosphate receptor type 1 |
|---|
| Ligand | BDBM50323707 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_645182 (CHEMBL1218398) |
|---|
| EC50 | 5±n/a nM |
|---|
| Citation |  Rossi, AM; Riley, AM; Tovey, SC; Rahman, T; Dellis, O; Taylor, EJ; Veresov, VG; Potter, BV; Taylor, CW Synthetic partial agonists reveal key steps in IP3 receptor activation. Nat Chem Biol5:631-9 (2009) [PubMed] Article Rossi, AM; Riley, AM; Tovey, SC; Rahman, T; Dellis, O; Taylor, EJ; Veresov, VG; Potter, BV; Taylor, CW Synthetic partial agonists reveal key steps in IP3 receptor activation. Nat Chem Biol5:631-9 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Inositol 1,4,5-trisphosphate receptor type 1 |
|---|
| Name: | Inositol 1,4,5-trisphosphate receptor type 1 |
|---|
| Synonyms: | ITPR1_RAT | Insp3r | Itpr1 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 313234.63 |
|---|
| Organism: | Rattus norvegicus |
|---|
| Description: | ChEMBL_818416 |
|---|
| Residue: | 2750 |
|---|
| Sequence: | MSDKMSSFLHIGDICSLYAEGSTNGFISTLGLVDDRCVVQPEAGDLNNPPKKFRDCLFKL
CPMNRYSAQKQFWKAAKPGANSTTDAVLLNKLHHAADLEKKQNETENRKLLGTVIQYGNV
IQLLHLKSNKYLTVNKRLPALLEKNAMRVTLDEAGNEGSWFYIQPFYKLRSIGDSVVIGD
KVVLNPVNAGQPLHASSHQLVDNPGCNEVNSVNCNTSWKIVLFMKWSDNKDDILKGGDVV
RLFHAEQEKFLTCDEHRKKQHVFLRTTGRQSATSATSSKALWEVEVVQHDPCRGGAGYWN
SLFRFKHLATGHYLAAEVDPDFEEECLEFQPSVDPDQDASRSRLRNAQEKMVYSLVSVPE
GNDISSIFELDPTTLRGGDSLVPRNSYVRLRHLCTNTWVHSTNIPIDKEEEKPVMLKIGT
SPLKEDKEAFAIVPVSPAEVRDLDFANDASKVLGSIAGKLEKGTITQNERRSVTKLLEDL
VYFVTGGTNSGQDVLEVVFSKPNRERQKLMREQNILKQIFKLLQAPFTDCGDGPMLRLEE
LGDQRHAPFRHICRLCYRVLRHSQQDYRKNQEYIAKQFGFMQKQIGYDVLAEDTITALLH
NNRKLLEKHITAAEIDTFVSLVRKNREPRFLDYLSDLCVSMNKSIPVTQELICKAVLNPT
NADILIETKLVLSRFEFEGVSTGENALEAGEDEEEVWLFWRDSNKEIRSKSVRELAQDAK
EGQKEDRDVLSYYRYQLNLFARMCLDRQYLAINEISGQLDVDLILRCMSDENLPYDLRAS
FCRLMLHMHVDRDPQEQVTPVKYARLWSEIPSEIAIDDYDSSGASKDEIKERFAQTMEFV
EEYLRDVVCQRFPFSDKEKNKLTFEVVNLARNLIYFGFYNFSDLLRLTKILLAILDCVHV
TTIFPISKMTKGEENKGSNVMRSIHGVGELMTQVVLRGGGFLPMTPMAAAPEGNVKQAEP
EKEDIMVMDTKLKIIEILQFILNVRLDYRISCLLCIFKREFDESNSQSSETSSGNSSQEG
PSNVPGALDFEHIEEQAEGIFGGSEENTPLDLDDHGGRTFLRVLLHLTMHDYPPLVSGAL
QLLFRHFSQRQEVLQAFKQVQLLVTSQDVDNYKQIKQDLDQLRSIVEKSELWVYKGQGPD
EPMDGASGENEHKKTEEGTSKPLKHESTSSYNYRVVKEILIRLSKLCVQESASVRKSRKQ
QQRLLRNMGAHAVVLELLQIPYEKAEDTKMQEIMRLAHEFLQNFCAGNQQNQALLHKHIN
LFLNPGILEAVTMQHIFMNNFQLCSEINERVVQHFVHCIETHGRNVQYIKFLQTIVKAEG
KFIKKCQDMVMAELVNSGEDVLVFYNDRASFQTLIQMMRSERDRMDENSPLFMYHIHLVE
LLAVCTEGKNVYTEIKCNSLLPLDDIVRVVTHEDCIPEVKIAYINFLNHCYVDTEVEMKE
IYTSNHMWKLFENFLVDICRACNNTSDRKHADSVLEKYVTEIVMSIVTTFFSSPFSDQST
TLQTRQPVFVQLLQGVFRVYHCNWLMPSQKASVESCIRVLSDVAKSRAIAIPVDLDSQVN
NLFLKSHNIVQKTAMNWRLSARNAARRDSVLAASRDYRNIIERLQDIVSALEDRLRPLVQ
AELSVLVDVLHRPELLFPENTDARRKCESGGFICKLIKHTKQLLEENEEKLCIKVLQTLR
EMMTKDRGYGEKQISIDELENAELPQPPEAENSTEQELEPSPPLRQLEDHKRGEALRQIL
VNRYYGNIRPSGRRESLTSFGNGPLSPGGPSKPGGGGGGPGSGSTSRGEMSLAEVQCHLD
KEGASNLVIDLIMNASSDRVFHESILLAIALLEGGNTTIQHSFFCRLTEDKKSEKFFKVF
YDRMKVAQQEIKATVTVNTSDLGNKKKDDEVDRDAPSRKKAKEPTTQITEEVRDQLLEAS
AATRKAFTTFRREADPDDHYQSGEGTQATTDKAKDDLEMSAVITIMQPILRFLQLLCENH
NRDLQNFLRCQNNKTNYNLVCETLQFLDCICGSTTGGLGLLGLYINEKNVALINQTLESL
TEYCQGPCHENQNCIATHESNGIDIITALILNDINPLGKKRMDLVLELKNNASKLLLAIM
ESRHDSENAERILYNMRPKELVEVIKKAYMQGEVEFEDGENGEDGAASPRNVGHNIYILA
HQLARHNKELQTMLKPGGQVDGDEALEFYAKHTAQIEIVRLDRTMEQIVFPVPSICEFLT
KESKLRIYYTTERDEQGSKINDFFLRSEDLFNEMNWQKKLRAQPVLYWCARNMSFWSSIS
FNLAVLMNLLVAFFYPFKGVRGGTLEPHWSGLLWTAMLISLAIVIALPKPHGIRALIAST
ILRLIFSVGLQPTLFLLGAFNVCNKIIFLMSFVGNCGTFTRGYRAMVLDVEFLYHLLYLL
ICAMGLFVHEFFYSLLLFDLVYREETLLNVIKSVTRNGRPIILTAALALILVYLFSIVGY
LFFKDDFILEVDRLPNETAGPETGESLANDFLYSDVCRVETGENCTSPAPKEELLPVEET
EQDKEHTCETLLMCIVTVLSHGLRSGGGVGDVLRKPSKEEPLFAARVIYDLLFFFMVIII
VLNLIFGVIIDTFADLRSEKQKKEEILKTTCFICGLERDKFDNKTVTFEEHIKEEHNMWH
YLCFIVLVKVKDSTEYTGPESYVAEMIRERNLDWFPRMRAMSLVSSDSEGEQNELRNLQE
KLESTMKLVTNLSGQLSELKDQMTEQRKQKQRIGLLGHPPHMNVNPQQPA
|
|
|
|---|
| BDBM50323707 |
|---|
| n/a |
|---|
| Name | BDBM50323707 |
|---|
| Synonyms: | CHEMBL1213160 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H42N2O37P8 |
|---|
| Mol. Mass. | 1114.2967 |
|---|
| SMILES | O[C@H]1[C@@H](OCCNC(=O)NCCO[C@@H]2[C@H](OP(O)(O)=O)[C@@H](OP(O)(O)=O)[C@H](OP(O)(O)O)[C@@H](OP(O)(O)=O)[C@@H]2OP(O)(O)=O)[C@@H](OP(O)(O)=O)[C@H](O)[C@@H](OP(O)(O)=O)[C@@H]1OP(O)(O)=O |r| |
|---|
| Structure | 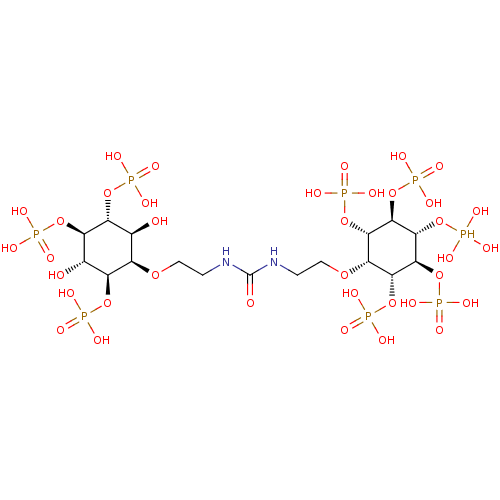
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Rossi, AM; Riley, AM; Tovey, SC; Rahman, T; Dellis, O; Taylor, EJ; Veresov, VG; Potter, BV; Taylor, CW Synthetic partial agonists reveal key steps in IP3 receptor activation. Nat Chem Biol5:631-9 (2009) [PubMed] Article
Rossi, AM; Riley, AM; Tovey, SC; Rahman, T; Dellis, O; Taylor, EJ; Veresov, VG; Potter, BV; Taylor, CW Synthetic partial agonists reveal key steps in IP3 receptor activation. Nat Chem Biol5:631-9 (2009) [PubMed] Article