 Found 699 hits with Last Name = 'potter' and Initial = 'bv'
Found 699 hits with Last Name = 'potter' and Initial = 'bv' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50532058
(CHEMBL4064554)Show SMILES COc1cc(CN2CCc3cc(OS(N)(=O)=O)ccc3C2)cc(OC)c1OC Show InChI InChI=1S/C19H24N2O6S/c1-24-17-8-13(9-18(25-2)19(17)26-3)11-21-7-6-14-10-16(27-28(20,22)23)5-4-15(14)12-21/h4-5,8-10H,6-7,11-12H2,1-3H3,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) expressed in Escherichia coli BL21 by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition against human carbonic anhydrase II |
Bioorg Med Chem Lett 14: 231-4 (2003)
BindingDB Entry DOI: 10.7270/Q2RN38DT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50134329

(CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition against human carbonic anhydrase II |
Bioorg Med Chem Lett 14: 231-4 (2003)
BindingDB Entry DOI: 10.7270/Q2RN38DT |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition against human carbonic anhydrase II |
Bioorg Med Chem Lett 14: 231-4 (2003)
BindingDB Entry DOI: 10.7270/Q2RN38DT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition against human carbonic anhydrase IX |
Bioorg Med Chem Lett 14: 231-4 (2003)
BindingDB Entry DOI: 10.7270/Q2RN38DT |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition against human carbonic anhydrase I |
Bioorg Med Chem Lett 14: 231-4 (2003)
BindingDB Entry DOI: 10.7270/Q2RN38DT |
More data for this
Ligand-Target Pair | |
Inositol 1,4,5-trisphosphate receptor type 3
(Bos taurus) | BDBM50132166

(1,2,4,5-InsP4 | CHEMBL100498 | Phosphoric acid mon...)Show SMILES OC1C(OP(O)(O)=O)C(OP(O)(O)=O)C(O)C(OP(O)(O)=O)C1OP(O)(O)=O |(-1.78,1.18,;-.25,1.18,;.51,2.53,;-.83,3.3,;-2.32,3.7,;-2.32,5.24,;-3.81,3.3,;-3.41,4.81,;2.14,1.91,;2.13,3.44,;1.73,4.93,;3.27,4.93,;.64,6.03,;2.13,6.44,;3.4,2.33,;3.4,3.88,;2.64,.99,;4.13,.6,;4.52,-.89,;6.06,-.89,;4.12,-2.37,;2.99,-.89,;.93,1.6,;.52,.12,;.52,-1.42,;-.97,-1.81,;1.28,-2.75,;.12,-2.89,)| Show InChI InChI=1S/C6H16O18P4/c7-1-3(21-25(9,10)11)5(23-27(15,16)17)2(8)6(24-28(18,19)20)4(1)22-26(12,13)14/h1-8H,(H2,9,10,11)(H2,12,13,14)(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 26.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to displace [3H]Ins(1,4,5)P3 from membranes prepared from bovine adrenal corticles |
Bioorg Med Chem Lett 3: 1505-1510 (1993)
Article DOI: 10.1016/S0960-894X(00)80007-4
BindingDB Entry DOI: 10.7270/Q27M08F4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50134329

(CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition against human carbonic anhydrase IX |
Bioorg Med Chem Lett 14: 231-4 (2003)
BindingDB Entry DOI: 10.7270/Q2RN38DT |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition against human carbonic anhydrase IX |
Bioorg Med Chem Lett 14: 231-4 (2003)
BindingDB Entry DOI: 10.7270/Q2RN38DT |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50134329

(CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition against human carbonic anhydrase I |
Bioorg Med Chem Lett 14: 231-4 (2003)
BindingDB Entry DOI: 10.7270/Q2RN38DT |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50200936
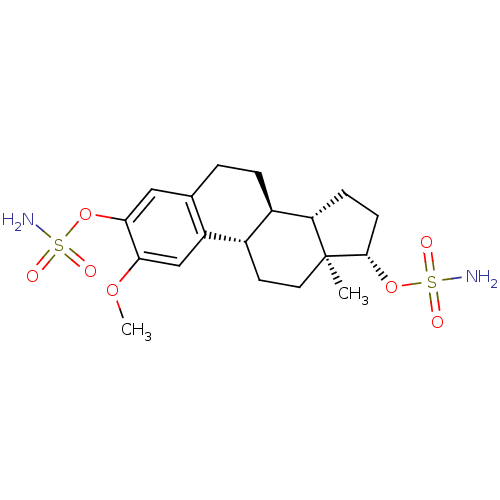
((9BETA,13ALPHA,14BETA,17ALPHA)-2-METHOXYESTRA-1,3,...)Show SMILES COc1cc2[C@H]3CC[C@]4(C)[C@H](CC[C@H]4[C@@H]3CCc2cc1OS(N)(=O)=O)OS(N)(=O)=O |r| Show InChI InChI=1S/C19H28N2O7S2/c1-19-8-7-12-13(15(19)5-6-18(19)28-30(21,24)25)4-3-11-9-17(27-29(20,22)23)16(26-2)10-14(11)12/h9-10,12-13,15,18H,3-8H2,1-2H3,(H2,20,22,23)(H2,21,24,25)/t12-,13+,15-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) expressed in Escherichia coli BL21 by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50532053
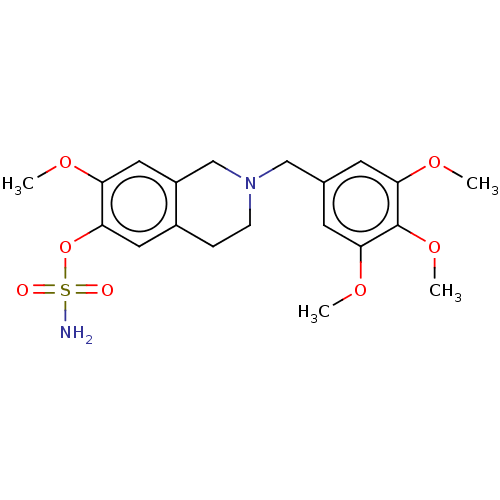
(CHEMBL3622044)Show SMILES COc1cc2CN(Cc3cc(OC)c(OC)c(OC)c3)CCc2cc1OS(N)(=O)=O Show InChI InChI=1S/C20H26N2O7S/c1-25-16-10-15-12-22(6-5-14(15)9-17(16)29-30(21,23)24)11-13-7-18(26-2)20(28-4)19(8-13)27-3/h7-10H,5-6,11-12H2,1-4H3,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) expressed in Escherichia coli BL21 by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50200941
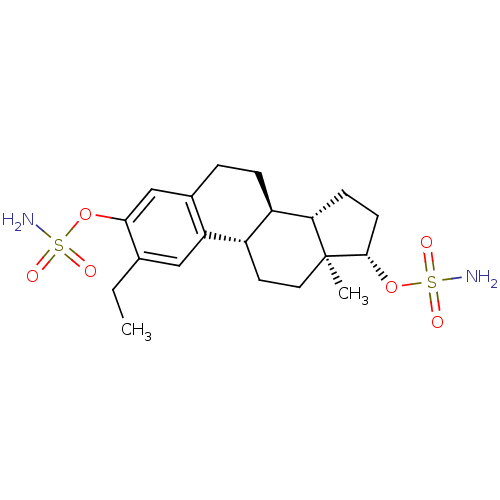
((9BETA,13ALPHA,14BETA,17ALPHA)-2-ETHYLESTRA-1(10),...)Show SMILES CCc1cc2[C@H]3CC[C@]4(C)[C@H](CC[C@H]4[C@@H]3CCc2cc1OS(N)(=O)=O)OS(N)(=O)=O |r| Show InChI InChI=1S/C20H30N2O6S2/c1-3-12-10-16-13(11-18(12)27-29(21,23)24)4-5-15-14(16)8-9-20(2)17(15)6-7-19(20)28-30(22,25)26/h10-11,14-15,17,19H,3-9H2,1-2H3,(H2,21,23,24)(H2,22,25,26)/t14-,15+,17-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 2 (unknown origin) |
J Med Chem 58: 7634-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00386
BindingDB Entry DOI: 10.7270/Q2474CP9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50200941

((9BETA,13ALPHA,14BETA,17ALPHA)-2-ETHYLESTRA-1(10),...)Show SMILES CCc1cc2[C@H]3CC[C@]4(C)[C@H](CC[C@H]4[C@@H]3CCc2cc1OS(N)(=O)=O)OS(N)(=O)=O |r| Show InChI InChI=1S/C20H30N2O6S2/c1-3-12-10-16-13(11-18(12)27-29(21,23)24)4-5-15-14(16)8-9-20(2)17(15)6-7-19(20)28-30(22,25)26/h10-11,14-15,17,19H,3-9H2,1-2H3,(H2,21,23,24)(H2,22,25,26)/t14-,15+,17-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) expressed in Escherichia coli BL21 by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50532057
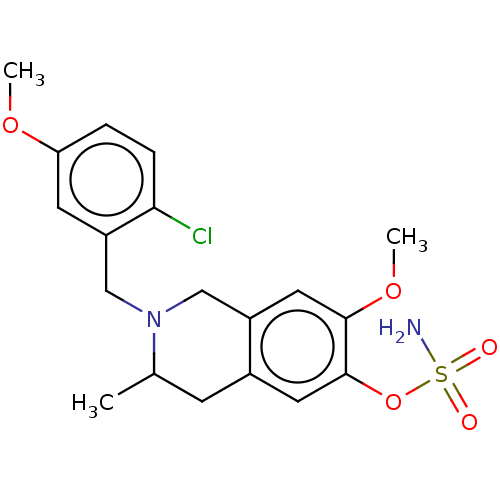
(CHEMBL4550943)Show SMILES COc1ccc(Cl)c(CN2Cc3cc(OC)c(OS(N)(=O)=O)cc3CC2C)c1 Show InChI InChI=1S/C19H23ClN2O5S/c1-12-6-13-8-19(27-28(21,23)24)18(26-3)9-14(13)10-22(12)11-15-7-16(25-2)4-5-17(15)20/h4-5,7-9,12H,6,10-11H2,1-3H3,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) expressed in Escherichia coli BL21 by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50200936

((9BETA,13ALPHA,14BETA,17ALPHA)-2-METHOXYESTRA-1,3,...)Show SMILES COc1cc2[C@H]3CC[C@]4(C)[C@H](CC[C@H]4[C@@H]3CCc2cc1OS(N)(=O)=O)OS(N)(=O)=O |r| Show InChI InChI=1S/C19H28N2O7S2/c1-19-8-7-12-13(15(19)5-6-18(19)28-30(21,24)25)4-3-11-9-17(27-29(20,22)23)16(26-2)10-14(11)12/h9-10,12-13,15,18H,3-8H2,1-2H3,(H2,20,22,23)(H2,21,24,25)/t12-,13+,15-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 2 (unknown origin) by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50532058

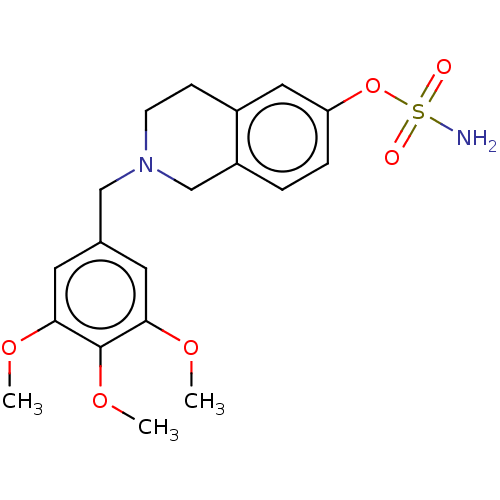
(CHEMBL4064554)Show SMILES COc1cc(CN2CCc3cc(OS(N)(=O)=O)ccc3C2)cc(OC)c1OC Show InChI InChI=1S/C19H24N2O6S/c1-24-17-8-13(9-18(25-2)19(17)26-3)11-21-7-6-14-10-16(27-28(20,22)23)5-4-15(14)12-21/h4-5,8-10H,6-7,11-12H2,1-3H3,(H2,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 2 (unknown origin) by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2
(Homo sapiens (Human)) | BDBM50469035

(CHEMBL4282693)Show InChI InChI=1S/C19H13Cl2F2NO2S/c20-12-5-4-11(14(21)8-12)10-26-17-6-7-27-18(17)19(25)24-9-13-15(22)2-1-3-16(13)23/h1-8H,9-10H2,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SHIP2 (419 to 732 residues) expressed in Escherichia coli by malachite green phosphate assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01944
BindingDB Entry DOI: 10.7270/Q2V98CZM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2
(Homo sapiens (Human)) | BDBM50430584

(CHEMBL2337806)Show SMILES C[C@H](NC(=O)c1sccc1OCc1ccc(Cl)cc1)c1ccccc1 |r| Show InChI InChI=1S/C20H18ClNO2S/c1-14(16-5-3-2-4-6-16)22-20(23)19-18(11-12-25-19)24-13-15-7-9-17(21)10-8-15/h2-12,14H,13H2,1H3,(H,22,23)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SHIP2 (419 to 732 residues) expressed in Escherichia coli by malachite green phosphate assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01944
BindingDB Entry DOI: 10.7270/Q2V98CZM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50532054
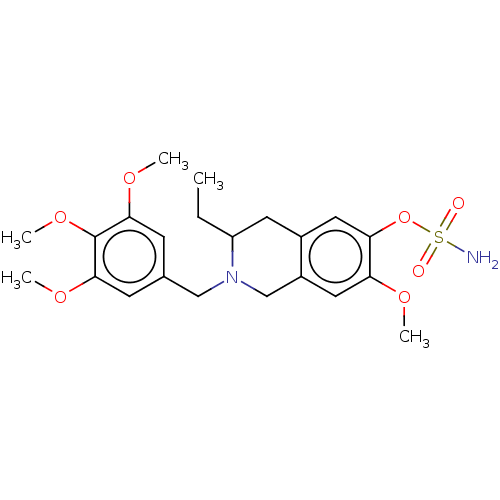
(CHEMBL4468079)Show SMILES CCC1Cc2cc(OS(N)(=O)=O)c(OC)cc2CN1Cc1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C22H30N2O7S/c1-6-17-9-15-10-19(31-32(23,25)26)18(27-2)11-16(15)13-24(17)12-14-7-20(28-3)22(30-5)21(8-14)29-4/h7-8,10-11,17H,6,9,12-13H2,1-5H3,(H2,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) expressed in Escherichia coli BL21 by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372895
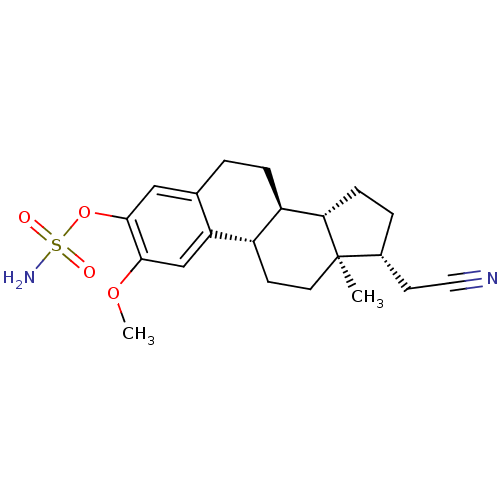
(CHEMBL408967)Show SMILES COc1cc2[C@H]3CC[C@]4(C)[C@@H](CC#N)CC[C@H]4[C@@H]3CCc2cc1OS(N)(=O)=O |r| Show InChI InChI=1S/C21H28N2O4S/c1-21-9-7-15-16(18(21)6-4-14(21)8-10-22)5-3-13-11-20(27-28(23,24)25)19(26-2)12-17(13)15/h11-12,14-16,18H,3-9H2,1-2H3,(H2,23,24,25)/t14-,15+,16-,18+,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) expressed in Escherichia coli BL21 by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50121070
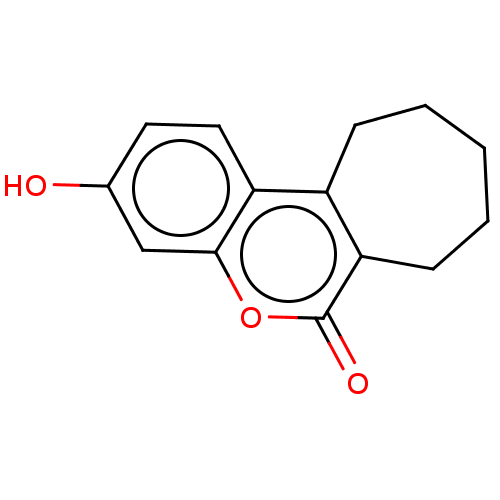
(CHEMBL3622017)Show InChI InChI=1S/C14H14O3/c15-9-6-7-11-10-4-2-1-3-5-12(10)14(16)17-13(11)8-9/h6-8,15H,1-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes |
J Med Chem 58: 7634-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00386
BindingDB Entry DOI: 10.7270/Q2474CP9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50532060
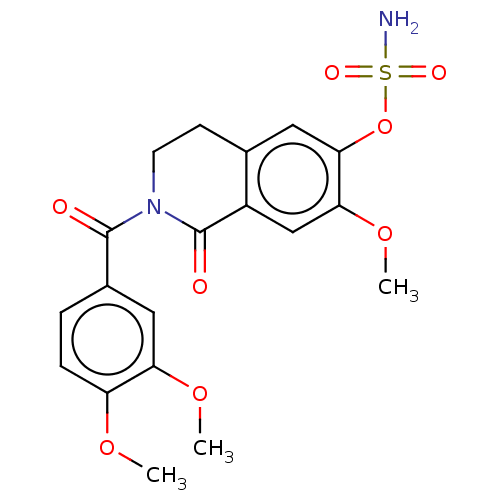
(CHEMBL4547186)Show SMILES COc1ccc(cc1OC)C(=O)N1CCc2cc(OS(N)(=O)=O)c(OC)cc2C1=O Show InChI InChI=1S/C19H20N2O8S/c1-26-14-5-4-12(9-15(14)27-2)18(22)21-7-6-11-8-17(29-30(20,24)25)16(28-3)10-13(11)19(21)23/h4-5,8-10H,6-7H2,1-3H3,(H2,20,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) expressed in Escherichia coli BL21 by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition against human carbonic anhydrase I |
Bioorg Med Chem Lett 14: 231-4 (2003)
BindingDB Entry DOI: 10.7270/Q2RN38DT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50532056
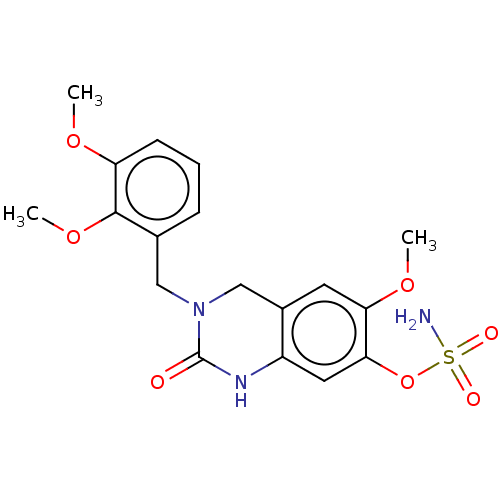
(CHEMBL4104342)Show SMILES COc1cccc(CN2Cc3cc(OC)c(OS(N)(=O)=O)cc3NC2=O)c1OC Show InChI InChI=1S/C18H21N3O7S/c1-25-14-6-4-5-11(17(14)27-3)9-21-10-12-7-15(26-2)16(28-29(19,23)24)8-13(12)20-18(21)22/h4-8H,9-10H2,1-3H3,(H,20,22)(H2,19,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) expressed in Escherichia coli BL21 by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50200940
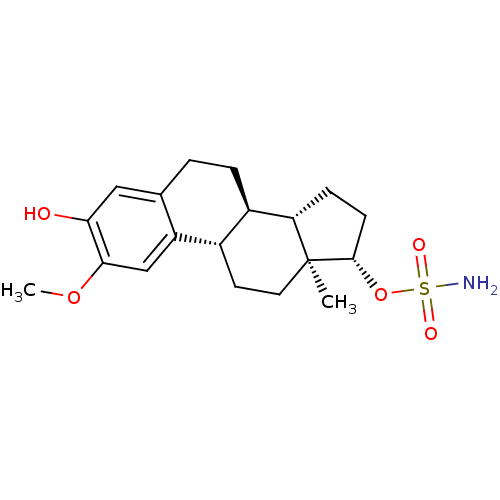
((13ALPHA,14BETA,17ALPHA)-3-HYDROXY-2-METHOXYESTRA-...)Show SMILES COc1cc2[C@H]3CC[C@]4(C)[C@H](CC[C@H]4[C@@H]3CCc2cc1O)OS(N)(=O)=O |r| Show InChI InChI=1S/C19H27NO5S/c1-19-8-7-12-13(15(19)5-6-18(19)25-26(20,22)23)4-3-11-9-16(21)17(24-2)10-14(11)12/h9-10,12-13,15,18,21H,3-8H2,1-2H3,(H2,20,22,23)/t12-,13+,15-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) expressed in Escherichia coli BL21 by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372895

(CHEMBL408967)Show SMILES COc1cc2[C@H]3CC[C@]4(C)[C@@H](CC#N)CC[C@H]4[C@@H]3CCc2cc1OS(N)(=O)=O |r| Show InChI InChI=1S/C21H28N2O4S/c1-21-9-7-15-16(18(21)6-4-14(21)8-10-22)5-3-13-11-20(27-28(23,24)25)19(26-2)12-17(13)15/h11-12,14-16,18H,3-9H2,1-2H3,(H2,23,24,25)/t14-,15+,16-,18+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 2 (unknown origin) by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50200941

((9BETA,13ALPHA,14BETA,17ALPHA)-2-ETHYLESTRA-1(10),...)Show SMILES CCc1cc2[C@H]3CC[C@]4(C)[C@H](CC[C@H]4[C@@H]3CCc2cc1OS(N)(=O)=O)OS(N)(=O)=O |r| Show InChI InChI=1S/C20H30N2O6S2/c1-3-12-10-16-13(11-18(12)27-29(21,23)24)4-5-15-14(16)8-9-20(2)17(15)6-7-19(20)28-30(22,25)26/h10-11,14-15,17,19H,3-9H2,1-2H3,(H2,21,23,24)(H2,22,25,26)/t14-,15+,17-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 2 (unknown origin) by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50532060

(CHEMBL4547186)Show SMILES COc1ccc(cc1OC)C(=O)N1CCc2cc(OS(N)(=O)=O)c(OC)cc2C1=O Show InChI InChI=1S/C19H20N2O8S/c1-26-14-5-4-12(9-15(14)27-2)18(22)21-7-6-11-8-17(29-30(20,24)25)16(28-3)10-13(11)19(21)23/h4-5,8-10H,6-7H2,1-3H3,(H2,20,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 2 (unknown origin) by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50532056

(CHEMBL4104342)Show SMILES COc1cccc(CN2Cc3cc(OC)c(OS(N)(=O)=O)cc3NC2=O)c1OC Show InChI InChI=1S/C18H21N3O7S/c1-25-14-6-4-5-11(17(14)27-3)9-21-10-12-7-15(26-2)16(28-29(19,23)24)8-13(12)20-18(21)22/h4-8H,9-10H2,1-3H3,(H,20,22)(H2,19,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 2 (unknown origin) by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2
(Homo sapiens (Human)) | BDBM50586357
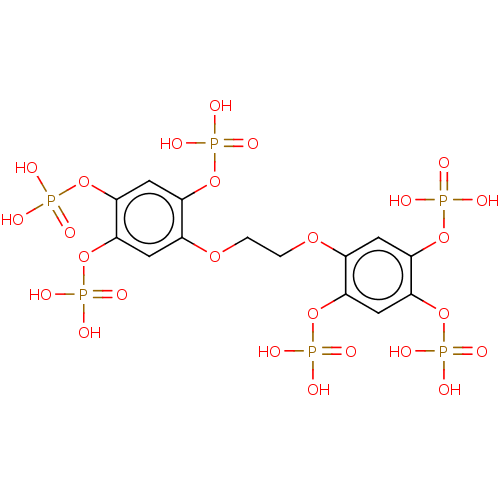
(CHEMBL5080660)Show SMILES OP(O)(=O)Oc1cc(OP(O)(O)=O)c(OP(O)(O)=O)cc1OCCOc1cc(OP(O)(O)=O)c(OP(O)(O)=O)cc1OP(O)(O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SHIP2 (419 to 732 residues) expressed in Escherichia coli by malachite green phosphate assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01944
BindingDB Entry DOI: 10.7270/Q2V98CZM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50532057

(CHEMBL4550943)Show SMILES COc1ccc(Cl)c(CN2Cc3cc(OC)c(OS(N)(=O)=O)cc3CC2C)c1 Show InChI InChI=1S/C19H23ClN2O5S/c1-12-6-13-8-19(27-28(21,23)24)18(26-3)9-14(13)10-22(12)11-15-7-16(25-2)4-5-17(15)20/h4-5,7-9,12H,6,10-11H2,1-3H3,(H2,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 2 (unknown origin) by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50532053

(CHEMBL3622044)Show SMILES COc1cc2CN(Cc3cc(OC)c(OC)c(OC)c3)CCc2cc1OS(N)(=O)=O Show InChI InChI=1S/C20H26N2O7S/c1-25-16-10-15-12-22(6-5-14(15)9-17(16)29-30(21,23)24)11-13-7-18(26-2)20(28-4)19(8-13)27-3/h7-10H,5-6,11-12H2,1-4H3,(H2,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 2 (unknown origin) by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50200940

((13ALPHA,14BETA,17ALPHA)-3-HYDROXY-2-METHOXYESTRA-...)Show SMILES COc1cc2[C@H]3CC[C@]4(C)[C@H](CC[C@H]4[C@@H]3CCc2cc1O)OS(N)(=O)=O |r| Show InChI InChI=1S/C19H27NO5S/c1-19-8-7-12-13(15(19)5-6-18(19)25-26(20,22)23)4-3-11-9-16(21)17(24-2)10-14(11)12/h9-10,12-13,15,18,21H,3-8H2,1-2H3,(H2,20,22,23)/t12-,13+,15-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 2 (unknown origin) by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50366322

(CHEMBL2367489)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@]1([H])c3ccc(O[P@@](C)(S)=O)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C19H25O3PS/c1-19-10-9-15-14-6-4-13(22-23(2,21)24)11-12(14)3-5-16(15)17(19)7-8-18(19)20/h4,6,11,15-17H,3,5,7-10H2,1-2H3,(H,21,24)/t15-,16-,17+,19+,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against estrone sulfatase in placental preparation |
Bioorg Med Chem Lett 3: 313-318 (1993)
Article DOI: 10.1016/S0960-894X(01)80900-8
BindingDB Entry DOI: 10.7270/Q2K074R2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50532055
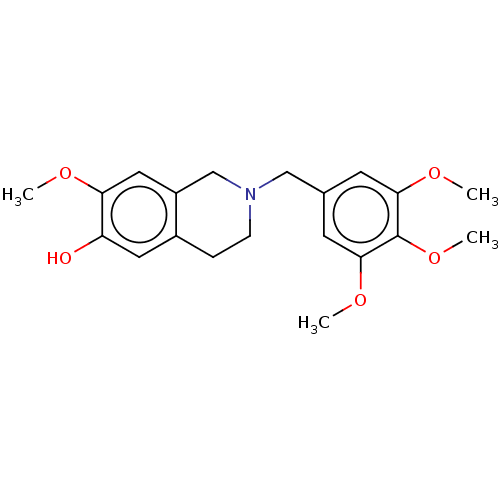
(CHEMBL4567341)Show InChI InChI=1S/C20H25NO5/c1-23-17-10-15-12-21(6-5-14(15)9-16(17)22)11-13-7-18(24-2)20(26-4)19(8-13)25-3/h7-10,22H,5-6,11-12H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) expressed in Escherichia coli BL21 by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Inositol polyphosphate-5-phosphatase A
(Homo sapiens (Human)) | BDBM50279840

((D)-2,2-difluoro-2-deoxy-myo-inositol 1,4,5-tripho...)Show SMILES OC1C(OP([O-])([O-])=O)C(OP([O-])([O-])=O)C(O)C(F)(F)C1OP([O-])([O-])=O Show InChI InChI=1S/C6H13F2O14P3/c7-6(8)4(10)3(21-24(14,15)16)2(20-23(11,12)13)1(9)5(6)22-25(17,18)19/h1-5,9-10H,(H2,11,12,13)(H2,14,15,16)(H2,17,18,19)/p-6 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity towards Inositol-1,4,5-trisphosphate 5-phosphatase |
Bioorg Med Chem Lett 1: 705-710 (1991)
Article DOI: 10.1016/S0960-894X(01)81052-0
BindingDB Entry DOI: 10.7270/Q2348KV6 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50366322

(CHEMBL2367489)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@]1([H])c3ccc(O[P@@](C)(S)=O)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C19H25O3PS/c1-19-10-9-15-14-6-4-13(22-23(2,21)24)11-12(14)3-5-16(15)17(19)7-8-18(19)20/h4,6,11,15-17H,3,5,7-10H2,1-2H3,(H,21,24)/t15-,16-,17+,19+,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against estrone sulfatase in breast tumor preparations |
Bioorg Med Chem Lett 3: 313-318 (1993)
Article DOI: 10.1016/S0960-894X(01)80900-8
BindingDB Entry DOI: 10.7270/Q2K074R2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50532055

(CHEMBL4567341)Show InChI InChI=1S/C20H25NO5/c1-23-17-10-15-12-21(6-5-14(15)9-16(17)22)11-13-7-18(24-2)20(26-4)19(8-13)25-3/h7-10,22H,5-6,11-12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 2 (unknown origin) by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50532054

(CHEMBL4468079)Show SMILES CCC1Cc2cc(OS(N)(=O)=O)c(OC)cc2CN1Cc1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C22H30N2O7S/c1-6-17-9-15-10-19(31-32(23,25)26)18(27-2)11-16(15)13-24(17)12-14-7-20(28-3)22(30-5)21(8-14)29-4/h7-8,10-11,17H,6,9,12-13H2,1-5H3,(H2,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 2 (unknown origin) by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50532059
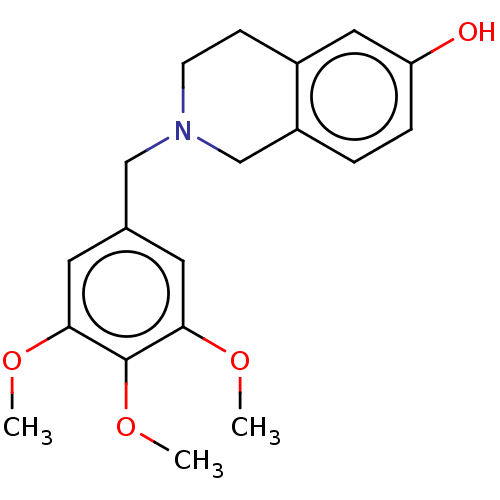
(CHEMBL4086075)Show InChI InChI=1S/C19H23NO4/c1-22-17-8-13(9-18(23-2)19(17)24-3)11-20-7-6-14-10-16(21)5-4-15(14)12-20/h4-5,8-10,21H,6-7,11-12H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) expressed in Escherichia coli BL21 by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Inositol 1,4,5-trisphosphate receptor type 1/2/3
(Homo sapiens (Human)) | BDBM50211666
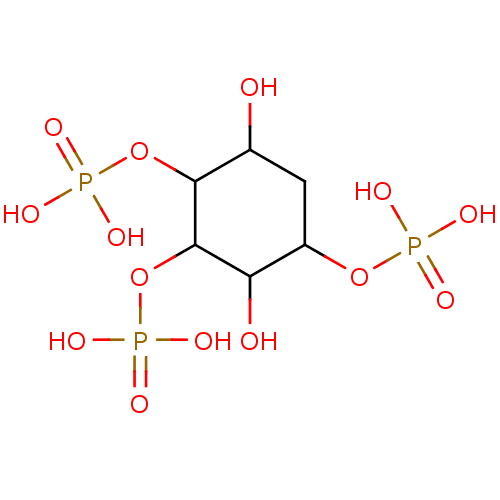
(CHEMBL1161456)Show SMILES OC1CC(OP(O)(O)=O)C(O)C(OP(O)(O)=O)C1OP(O)(O)=O Show InChI InChI=1S/C6H15O14P3/c7-2-1-3(18-21(9,10)11)4(8)6(20-23(15,16)17)5(2)19-22(12,13)14/h2-8H,1H2,(H2,9,10,11)(H2,12,13,14)(H2,15,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 2.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to inhibit Inositol phosphorylation |
Bioorg Med Chem Lett 1: 705-710 (1991)
Article DOI: 10.1016/S0960-894X(01)81052-0
BindingDB Entry DOI: 10.7270/Q2348KV6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50532059

(CHEMBL4086075)Show InChI InChI=1S/C19H23NO4/c1-22-17-8-13(9-18(23-2)19(17)24-3)11-20-7-6-14-10-16(21)5-4-15(14)12-20/h4-5,8-10,21H,6-7,11-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 2 (unknown origin) by stopped-flow CO2 hydration assay |
J Med Chem 62: 2202-2212 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01990
BindingDB Entry DOI: 10.7270/Q2765JSP |
More data for this
Ligand-Target Pair | |
Inositol-trisphosphate 3-kinase C
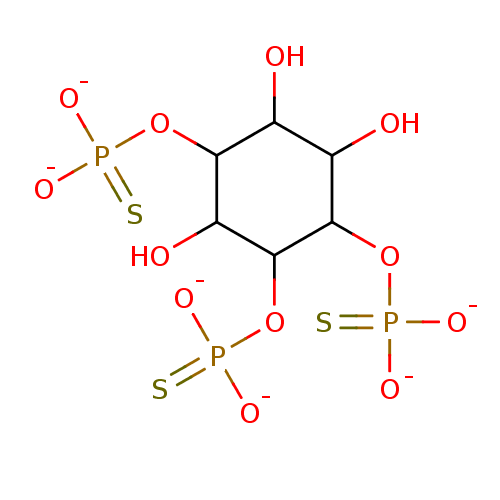
(Rattus norvegicus) | BDBM50040911
((+)-myo-inositol 1,4,5-trisphosphorothioate | (-)-...)Show SMILES OC1C(O)C(OP([O-])([O-])=S)C(OP([O-])([O-])=S)C(O)C1OP([O-])([O-])=S Show InChI InChI=1S/C6H15O12P3S3/c7-1-2(8)5(17-20(12,13)23)6(18-21(14,15)24)3(9)4(1)16-19(10,11)22/h1-9H,(H2,10,11,22)(H2,12,13,23)(H2,14,15,24)/p-6 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 9.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the ability to inhibit Ins(1,4,5)P 3-phosphatase |
Bioorg Med Chem Lett 2: 1523-1528 (1992)
Article DOI: 10.1016/S0960-894X(00)80421-7
BindingDB Entry DOI: 10.7270/Q2NZ8849 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase type 2
(Rattus norvegicus) | BDBM50147505
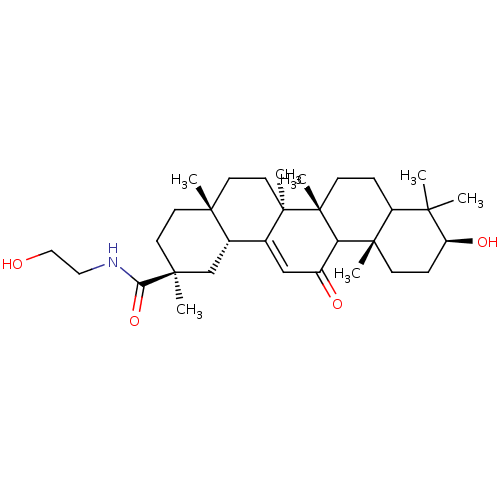
((2S,4aS,6aS,6bR,10S,12aS,14bR)-10-Hydroxy-2,4a,6a,...)Show SMILES CC1(C)[C@@H](O)CC[C@@]2(C)C1CC[C@]1(C)C2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(=O)NCCO |t:19| Show InChI InChI=1S/C32H51NO4/c1-27(2)23-8-11-32(7)25(30(23,5)10-9-24(27)36)22(35)18-20-21-19-29(4,26(37)33-16-17-34)13-12-28(21,3)14-15-31(20,32)6/h18,21,23-25,34,36H,8-17,19H2,1-7H3,(H,33,37)/t21-,23?,24-,25?,28+,29-,30-,31+,32+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Percent inhibition against 11-beta-hydroxysteroid dehydrogenase 2 of wistar rat kidney at 10 microM was determined using [3H]-cortisol |
Bioorg Med Chem Lett 14: 3263-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.107
BindingDB Entry DOI: 10.7270/Q2H41QWC |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50380208

(CHEMBL2011408)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4c3ccc(OS(N)(=O)=O)c4[N+]([O-])=O)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C18H22N2O6S/c1-18-9-8-11-10-4-6-15(26-27(19,24)25)17(20(22)23)13(10)3-2-12(11)14(18)5-7-16(18)21/h4,6,11-12,14H,2-3,5,7-9H2,1H3,(H2,19,24,25)/t11-,12-,14+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human MCF7 cells using [3H]E1S as substrate after 20 hrs by scintillation spectrometry |
Bioorg Med Chem 20: 2506-19 (2012)
Article DOI: 10.1016/j.bmc.2012.03.007
BindingDB Entry DOI: 10.7270/Q2XG9S4J |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50121196
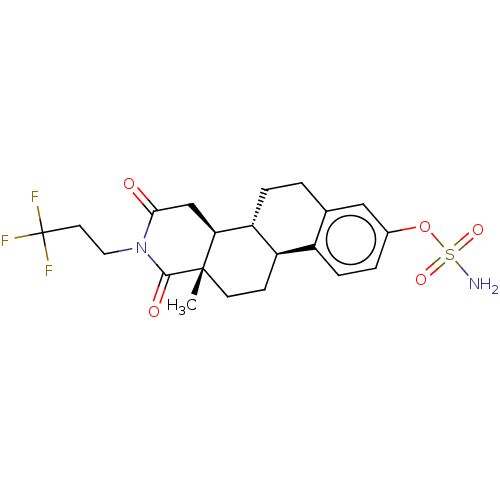
(CHEMBL3622029)Show SMILES [H][C@]12CC[C@]3(C)C(=O)N(CCC(F)(F)F)C(=O)C[C@@]3([H])[C@]1([H])CCc1cc(OS(N)(=O)=O)ccc21 |r| Show InChI InChI=1S/C21H25F3N2O5S/c1-20-7-6-15-14-5-3-13(31-32(25,29)30)10-12(14)2-4-16(15)17(20)11-18(27)26(19(20)28)9-8-21(22,23)24/h3,5,10,15-17H,2,4,6-9,11H2,1H3,(H2,25,29,30)/t15-,16-,17+,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of STS activity in human JEG-3 cells by liquid scintillation spectrometry in presence of [6,7-3H]estrone3-sulfate |
J Med Chem 58: 7634-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00386
BindingDB Entry DOI: 10.7270/Q2474CP9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50380223

(CHEMBL2011422)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)c(cc34)C(F)F)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H23F2NO4S/c1-19-7-6-11-12(15(19)4-5-17(19)23)3-2-10-8-16(26-27(22,24)25)14(18(20)21)9-13(10)11/h8-9,11-12,15,18H,2-7H2,1H3,(H2,22,24,25)/t11-,12+,15-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human placental microsomes using [3H]E1S as substrate after 30 mins by scintillation spectrometry |
Bioorg Med Chem 20: 2506-19 (2012)
Article DOI: 10.1016/j.bmc.2012.03.007
BindingDB Entry DOI: 10.7270/Q2XG9S4J |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50307918

(2-(5-((1H-1,2,4-triazol-1-yl)methyl)-3'-chloro-4'-...)Show SMILES CC(C)(C#N)c1cc(Cn2cncn2)cc(c1)-c1ccc(O)c(Cl)c1 Show InChI InChI=1S/C19H17ClN4O/c1-19(2,10-21)16-6-13(9-24-12-22-11-23-24)5-15(7-16)14-3-4-18(25)17(20)8-14/h3-8,11-12,25H,9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatse in human JEG3 cells by scintillation spectrometry |
J Med Chem 53: 2155-70 (2010)
Article DOI: 10.1021/jm901705h
BindingDB Entry DOI: 10.7270/Q2959JGF |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50380216
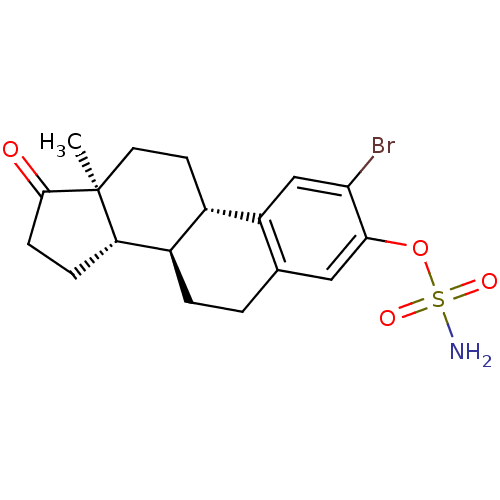
(CHEMBL364332)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)c(Br)cc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H22BrNO4S/c1-18-7-6-11-12(14(18)4-5-17(18)21)3-2-10-8-16(24-25(20,22)23)15(19)9-13(10)11/h8-9,11-12,14H,2-7H2,1H3,(H2,20,22,23)/t11-,12+,14-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human MCF7 cells using [3H]E1S as substrate after 20 hrs by scintillation spectrometry |
Bioorg Med Chem 20: 2506-19 (2012)
Article DOI: 10.1016/j.bmc.2012.03.007
BindingDB Entry DOI: 10.7270/Q2XG9S4J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data