 Found 6 hits for monomerid = 50326169
Found 6 hits for monomerid = 50326169 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-Dependent Kinase 1 (CDK1)
(Homo sapiens (Human)) | BDBM50326169
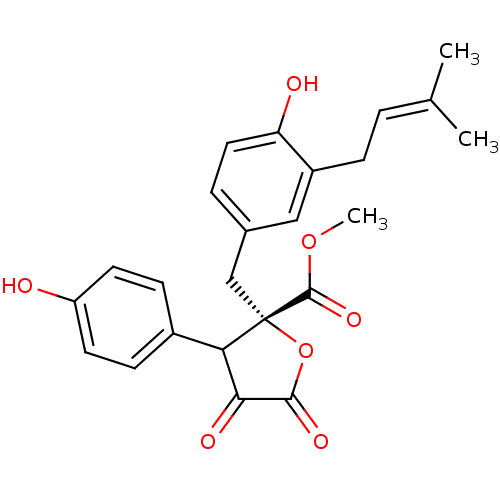
((S)-methyl 4-hydroxy-2-(4-hydroxy-3-(3-methylbut-2...)Show SMILES [#6]-[#8]-[#6](=O)[C@@]1([#6]-c2ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2)[#8]-[#6](=O)-[#6](=O)-[#6]1-c1ccc(-[#8])cc1 |r| Show InChI InChI=1S/C24H24O7/c1-14(2)4-6-17-12-15(5-11-19(17)26)13-24(23(29)30-3)20(21(27)22(28)31-24)16-7-9-18(25)10-8-16/h4-5,7-12,20,25-26H,6,13H2,1-3H3/t20?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human GST-CDK1/cyclin B1 expressed in baculovirus using [gamma-33P]ATP after 45 mins by liquid scintillation counting |
Eur J Med Chem 45: 4316-30 (2010)
Article DOI: 10.1016/j.ejmech.2010.06.034
BindingDB Entry DOI: 10.7270/Q2W66KZM |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50326169

((S)-methyl 4-hydroxy-2-(4-hydroxy-3-(3-methylbut-2...)Show SMILES [#6]-[#8]-[#6](=O)[C@@]1([#6]-c2ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2)[#8]-[#6](=O)-[#6](=O)-[#6]1-c1ccc(-[#8])cc1 |r| Show InChI InChI=1S/C24H24O7/c1-14(2)4-6-17-12-15(5-11-19(17)26)13-24(23(29)30-3)20(21(27)22(28)31-24)16-7-9-18(25)10-8-16/h4-5,7-12,20,25-26H,6,13H2,1-3H3/t20?,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
AAPS J 8: 204-21 (2006)
Article DOI: 10.1208/aapsj080125
BindingDB Entry DOI: 10.7270/Q2TX3GBS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50326169

((S)-methyl 4-hydroxy-2-(4-hydroxy-3-(3-methylbut-2...)Show SMILES [#6]-[#8]-[#6](=O)[C@@]1([#6]-c2ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2)[#8]-[#6](=O)-[#6](=O)-[#6]1-c1ccc(-[#8])cc1 |r| Show InChI InChI=1S/C24H24O7/c1-14(2)4-6-17-12-15(5-11-19(17)26)13-24(23(29)30-3)20(21(27)22(28)31-24)16-7-9-18(25)10-8-16/h4-5,7-12,20,25-26H,6,13H2,1-3H3/t20?,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
AAPS J 8: 204-21 (2006)
Article DOI: 10.1208/aapsj080125
BindingDB Entry DOI: 10.7270/Q2TX3GBS |
More data for this
Ligand-Target Pair | |
Dual-specificity tyrosine-phosphorylation regulated kinase 1A
(Homo sapiens (Human)) | BDBM50326169

((S)-methyl 4-hydroxy-2-(4-hydroxy-3-(3-methylbut-2...)Show SMILES [#6]-[#8]-[#6](=O)[C@@]1([#6]-c2ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2)[#8]-[#6](=O)-[#6](=O)-[#6]1-c1ccc(-[#8])cc1 |r| Show InChI InChI=1S/C24H24O7/c1-14(2)4-6-17-12-15(5-11-19(17)26)13-24(23(29)30-3)20(21(27)22(28)31-24)16-7-9-18(25)10-8-16/h4-5,7-12,20,25-26H,6,13H2,1-3H3/t20?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leibniz-Institute for Natural Product Research and Infection Biology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DYRK1A |
J Nat Prod 71: 689-92 (2008)
Article DOI: 10.1021/np070341r
BindingDB Entry DOI: 10.7270/Q2154HZX |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50326169

((S)-methyl 4-hydroxy-2-(4-hydroxy-3-(3-methylbut-2...)Show SMILES [#6]-[#8]-[#6](=O)[C@@]1([#6]-c2ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2)[#8]-[#6](=O)-[#6](=O)-[#6]1-c1ccc(-[#8])cc1 |r| Show InChI InChI=1S/C24H24O7/c1-14(2)4-6-17-12-15(5-11-19(17)26)13-24(23(29)30-3)20(21(27)22(28)31-24)16-7-9-18(25)10-8-16/h4-5,7-12,20,25-26H,6,13H2,1-3H3/t20?,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
AAPS J 8: 204-21 (2006)
Article DOI: 10.1208/aapsj080125
BindingDB Entry DOI: 10.7270/Q2TX3GBS |
More data for this
Ligand-Target Pair | |
Protein kinase Pfmrk
(Plasmodium falciparum) | BDBM50326169

((S)-methyl 4-hydroxy-2-(4-hydroxy-3-(3-methylbut-2...)Show SMILES [#6]-[#8]-[#6](=O)[C@@]1([#6]-c2ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2)[#8]-[#6](=O)-[#6](=O)-[#6]1-c1ccc(-[#8])cc1 |r| Show InChI InChI=1S/C24H24O7/c1-14(2)4-6-17-12-15(5-11-19(17)26)13-24(23(29)30-3)20(21(27)22(28)31-24)16-7-9-18(25)10-8-16/h4-5,7-12,20,25-26H,6,13H2,1-3H3/t20?,24-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plasmodium falciparum cyclin dependent protein kinase, Pfmrk |
J Med Chem 46: 3877-82 (2003)
Article DOI: 10.1021/jm0300983
BindingDB Entry DOI: 10.7270/Q2028S9W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data