

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50326985 3,3'-((2Z,2'Z)-4,4'-(3,4-dimethoxyphenethylazanediyl)bis(but-2-ene-4,1-diyl))bis(7,8-dimethoxy-1H-benzo[d]azepin-2(3H)-one)::CHEMBL1253477
SMILES: COc1ccc(CCN(C\C=C/CN2C=Cc3cc(OC)c(OC)cc3CC2=O)C\C=C/CN2C=Cc3cc(OC)c(OC)cc3CC2=O)cc1OC
InChI Key: InChIKey=HYAMGVKMAKVYBM-XOHWUJONSA-N
Data: 3 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 2 (Mus musculus) | BDBM50326985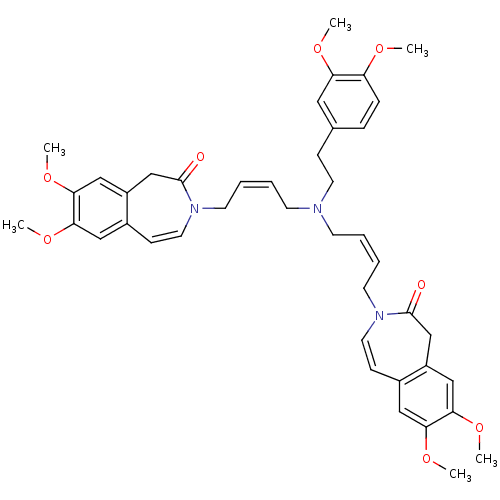 (3,3'-((2Z,2'Z)-4,4'-(3,4-dimethoxyphenethylazanedi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.47E+5 | n/a | n/a | n/a | n/a |
Laboratory of Design, Synthesis, and Study of Biologically Active Heterocycles (HeteroBioLab) Curated by ChEMBL | Assay Description Blockade of mouse HCN2 expressed in HEK293 cells at -120 f-current amplitude by patch-clamp electrophysiological assay | J Med Chem 53: 6773-7 (2010) Article DOI: 10.1021/jm1006758 BindingDB Entry DOI: 10.7270/Q2T43T9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 4 (Homo sapiens (Human)) | BDBM50326985 (3,3'-((2Z,2'Z)-4,4'-(3,4-dimethoxyphenethylazanedi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.07E+4 | n/a | n/a | n/a | n/a |
Laboratory of Design, Synthesis, and Study of Biologically Active Heterocycles (HeteroBioLab) Curated by ChEMBL | Assay Description Blockade of human HCN4 expressed in HEK293 cells at -120 f-current amplitude by patch-clamp electrophysiological assay | J Med Chem 53: 6773-7 (2010) Article DOI: 10.1021/jm1006758 BindingDB Entry DOI: 10.7270/Q2T43T9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 1 (Mus musculus) | BDBM50326985 (3,3'-((2Z,2'Z)-4,4'-(3,4-dimethoxyphenethylazanedi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.07E+5 | n/a | n/a | n/a | n/a |
Laboratory of Design, Synthesis, and Study of Biologically Active Heterocycles (HeteroBioLab) Curated by ChEMBL | Assay Description Blockade of mouse HCN1 expressed in HEK293 cells at -120 f-current amplitude by patch-clamp electrophysiological assay | J Med Chem 53: 6773-7 (2010) Article DOI: 10.1021/jm1006758 BindingDB Entry DOI: 10.7270/Q2T43T9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||