

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50327504 1,4-Dideoxy-1,4-[[2S,3S,4R,5S]-2,4,5,6-tetrahydroxy-3-(methyl)hexyl]-(R)-epi-sulfoniumylidine]-D-arabinitol chloride::CHEMBL1258644
SMILES: CO[C@H]([C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO)[C@H](O)[C@@H](O)CO
InChI Key: InChIKey=ZFLJGAPPQIUCTG-DUJHBOIWSA-N
Data: 5 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sucrase-isomaltase (Homo sapiens (Human)) | BDBM50327504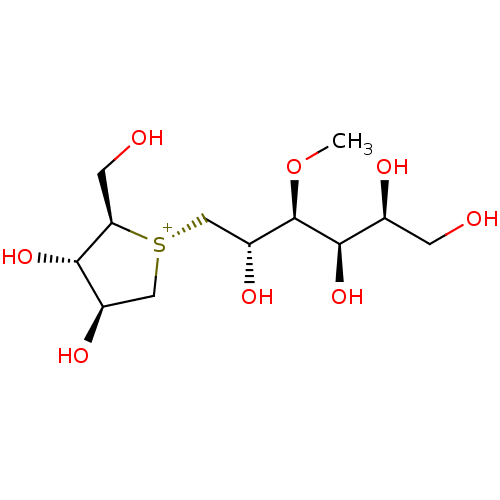 (1,4-Dideoxy-1,4-[[2S,3S,4R,5S]-2,4,5,6-tetrahydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Homo sapiens (Human)) | BDBM50327504 (1,4-Dideoxy-1,4-[[2S,3S,4R,5S]-2,4,5,6-tetrahydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50327504 (1,4-Dideoxy-1,4-[[2S,3S,4R,5S]-2,4,5,6-tetrahydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human N-terminal maltase-glucoamylase | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50327504 (1,4-Dideoxy-1,4-[[2S,3S,4R,5S]-2,4,5,6-tetrahydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal maltase-glucoamylase by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50327504 (1,4-Dideoxy-1,4-[[2S,3S,4R,5S]-2,4,5,6-tetrahydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal domain of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem Lett 20: 5686-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.020 BindingDB Entry DOI: 10.7270/Q2WQ0411 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||