 Found 8 hits for monomerid = 50329070
Found 8 hits for monomerid = 50329070 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329070
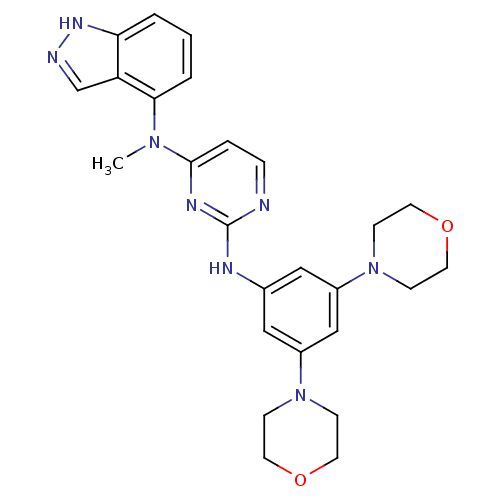
(CHEMBL1269858 | N2-(3,5-dimorpholinophenyl)-N4-(1H...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H30N8O2/c1-32(24-4-2-3-23-22(24)18-28-31-23)25-5-6-27-26(30-25)29-19-15-20(33-7-11-35-12-8-33)17-21(16-19)34-9-13-36-14-10-34/h2-6,15-18H,7-14H2,1H3,(H,28,31)(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329070

(CHEMBL1269858 | N2-(3,5-dimorpholinophenyl)-N4-(1H...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H30N8O2/c1-32(24-4-2-3-23-22(24)18-28-31-23)25-5-6-27-26(30-25)29-19-15-20(33-7-11-35-12-8-33)17-21(16-19)34-9-13-36-14-10-34/h2-6,15-18H,7-14H2,1H3,(H,28,31)(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 autophosphorylation expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50329070

(CHEMBL1269858 | N2-(3,5-dimorpholinophenyl)-N4-(1H...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H30N8O2/c1-32(24-4-2-3-23-22(24)18-28-31-23)25-5-6-27-26(30-25)29-19-15-20(33-7-11-35-12-8-33)17-21(16-19)34-9-13-36-14-10-34/h2-6,15-18H,7-14H2,1H3,(H,28,31)(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of KDR phosphorylation in HUVEC |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50329070

(CHEMBL1269858 | N2-(3,5-dimorpholinophenyl)-N4-(1H...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H30N8O2/c1-32(24-4-2-3-23-22(24)18-28-31-23)25-5-6-27-26(30-25)29-19-15-20(33-7-11-35-12-8-33)17-21(16-19)34-9-13-36-14-10-34/h2-6,15-18H,7-14H2,1H3,(H,28,31)(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes by fluorometric assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50329070

(CHEMBL1269858 | N2-(3,5-dimorpholinophenyl)-N4-(1H...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H30N8O2/c1-32(24-4-2-3-23-22(24)18-28-31-23)25-5-6-27-26(30-25)29-19-15-20(33-7-11-35-12-8-33)17-21(16-19)34-9-13-36-14-10-34/h2-6,15-18H,7-14H2,1H3,(H,28,31)(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes by fluorometric assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50329070

(CHEMBL1269858 | N2-(3,5-dimorpholinophenyl)-N4-(1H...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H30N8O2/c1-32(24-4-2-3-23-22(24)18-28-31-23)25-5-6-27-26(30-25)29-19-15-20(33-7-11-35-12-8-33)17-21(16-19)34-9-13-36-14-10-34/h2-6,15-18H,7-14H2,1H3,(H,28,31)(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes by fluorometric assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50329070

(CHEMBL1269858 | N2-(3,5-dimorpholinophenyl)-N4-(1H...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H30N8O2/c1-32(24-4-2-3-23-22(24)18-28-31-23)25-5-6-27-26(30-25)29-19-15-20(33-7-11-35-12-8-33)17-21(16-19)34-9-13-36-14-10-34/h2-6,15-18H,7-14H2,1H3,(H,28,31)(H,27,29,30) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes by fluorometric assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50329070

(CHEMBL1269858 | N2-(3,5-dimorpholinophenyl)-N4-(1H...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H30N8O2/c1-32(24-4-2-3-23-22(24)18-28-31-23)25-5-6-27-26(30-25)29-19-15-20(33-7-11-35-12-8-33)17-21(16-19)34-9-13-36-14-10-34/h2-6,15-18H,7-14H2,1H3,(H,28,31)(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta phosphorylation in human MG63 cells |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data