

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCc1cc2OCOc2cc1Cn1c(C(O)=O)c(Cc2cccc(c2)C(O)=O)c(=O)c2cc(OCC(C)C)ccc12
InChI Key: InChIKey=ASYDIUHFIWWNJR-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50329844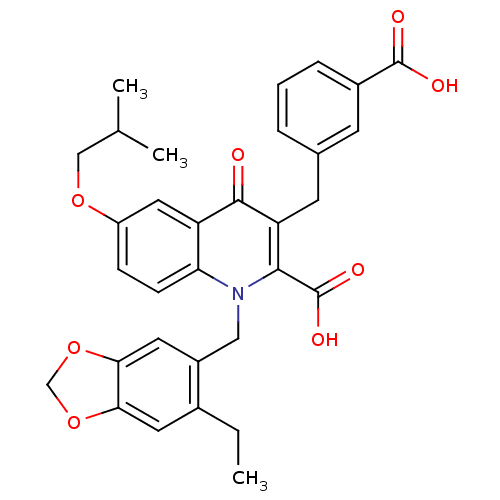 (3-(3-Carboxybenzyl)-1-[(6-ethylbenzo[d][1,3]dioxol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
St. John's University Curated by ChEMBL | Assay Description Displacement of [125I]ET1 from ETA receptor expressed in african green monkey CCL-81 cells monolayer | Bioorg Med Chem Lett 20: 6840-4 (2010) Article DOI: 10.1016/j.bmcl.2010.08.074 BindingDB Entry DOI: 10.7270/Q2X34XPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50329844 (3-(3-Carboxybenzyl)-1-[(6-ethylbenzo[d][1,3]dioxol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 713 | n/a | n/a | n/a | n/a | n/a | n/a |
St. John's University Curated by ChEMBL | Assay Description Antagonist activity at ETB receptor (unknown origin) expressed in HEK293T cells measured after 30 mins by CCF4-AM dye based GeneBlazer FRET assay | Bioorg Med Chem Lett 27: 2281-2285 (2017) Article DOI: 10.1016/j.bmcl.2017.04.049 BindingDB Entry DOI: 10.7270/Q2Q52S21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50329844 (3-(3-Carboxybenzyl)-1-[(6-ethylbenzo[d][1,3]dioxol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
St. John's University Curated by ChEMBL | Assay Description Antagonist activity at ETA receptor (unknown origin) expressed in membranes by radioligand assay | Bioorg Med Chem Lett 27: 2281-2285 (2017) Article DOI: 10.1016/j.bmcl.2017.04.049 BindingDB Entry DOI: 10.7270/Q2Q52S21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50329844 (3-(3-Carboxybenzyl)-1-[(6-ethylbenzo[d][1,3]dioxol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. John's University Curated by ChEMBL | Assay Description Displacement of [125I]ET1 from human ETB receptor expressed in membranes | Bioorg Med Chem Lett 20: 6840-4 (2010) Article DOI: 10.1016/j.bmcl.2010.08.074 BindingDB Entry DOI: 10.7270/Q2X34XPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||