

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50330319 (3R,4S,5R,6S)-6-(8-chloro-4-hydroxy-3-(4-hydroxy-3,5-dimethylbenzamido)-2-oxo-2H-chromen-7-yloxy)-5-hydroxy-3-methoxy-2,2-dimethyltetrahydro-2H-pyran-4-yl 5-methyl-1H-pyrrole-2-carboxylate::CHEMBL1275840
SMILES: CO[C@@H]1[C@@H](OC(=O)c2ccc(C)[nH]2)[C@@H](O)[C@H](Oc2ccc3c(O)c(NC(=O)c4cc(C)c(O)c(C)c4)c(=O)oc3c2Cl)OC1(C)C
InChI Key: InChIKey=DOFGTZAYJOIEPN-PPOHMGSZSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA Gyrase Subunit B (Escherichia coli (strain K12)) | BDBM50330319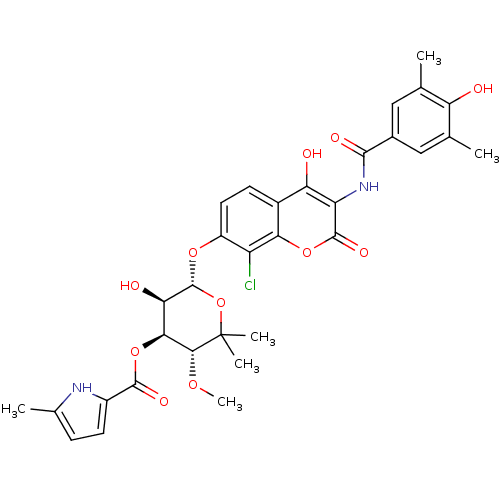 ((3R,4S,5R,6S)-6-(8-chloro-4-hydroxy-3-(4-hydroxy-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Tübingen Curated by ChEMBL | Assay Description Inhibition of Escherichia coli JM109 DNA gyrase subunit B assessed as DNA triple helix formation by supercoiling assay | Antimicrob Agents Chemother 52: 1982-90 (2008) Article DOI: 10.1128/AAC.01235-07 BindingDB Entry DOI: 10.7270/Q2PG1S0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA Gyrase Subunit B (Escherichia coli (strain K12)) | BDBM50330319 ((3R,4S,5R,6S)-6-(8-chloro-4-hydroxy-3-(4-hydroxy-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Tübingen Curated by ChEMBL | Assay Description Inhibition of Escherichia coli JM109 DNA gyrase subunit B assessed as ATP hydrolysis by ATPase assay | Antimicrob Agents Chemother 52: 1982-90 (2008) Article DOI: 10.1128/AAC.01235-07 BindingDB Entry DOI: 10.7270/Q2PG1S0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||