

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50336753 4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-methoxyphenoxy]-3-chlorobenzoic Acid Methyl Ester::CHEMBL1671964
SMILES: COC(=O)c1ccc(Oc2ccc(C=C3SC(O)=NC3=O)cc2OC)c(Cl)c1
InChI Key: InChIKey=JATLXGAUZPZTKI-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336753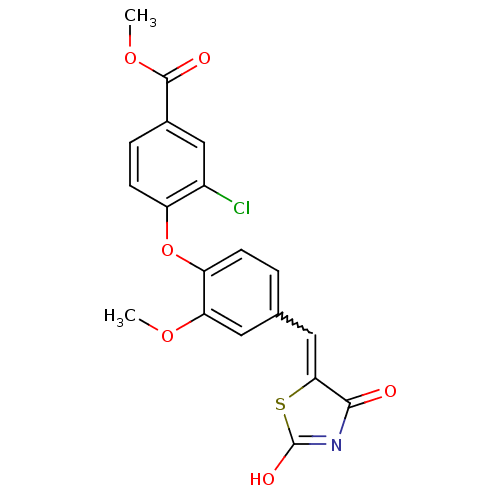 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50336753 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50336753 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336753 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||