 Found 14 hits for monomerid = 50337278
Found 14 hits for monomerid = 50337278 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50337278
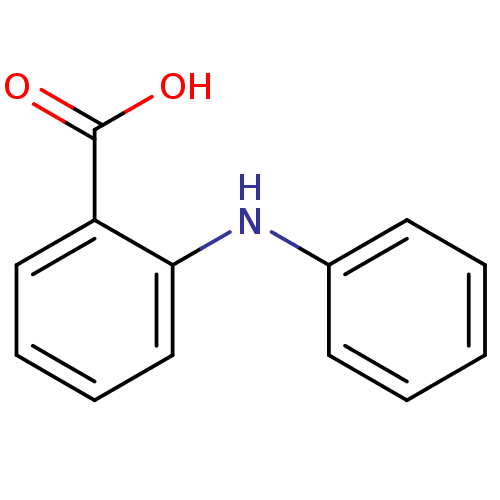
(2-(phenylamino)benzoic acid | 2-Phenylamino-benzoi...)Show InChI InChI=1S/C13H11NO2/c15-13(16)11-8-4-5-9-12(11)14-10-6-2-1-3-7-10/h1-9,14H,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using CO2 as substrate incubated for 6 hrs prior to substrate addition measured for 10 to 100 secs by stopped flow technique |
Bioorg Med Chem Lett 22: 5801-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.094
BindingDB Entry DOI: 10.7270/Q2319X0G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50337278

(2-(phenylamino)benzoic acid | 2-Phenylamino-benzoi...)Show InChI InChI=1S/C13H11NO2/c15-13(16)11-8-4-5-9-12(11)14-10-6-2-1-3-7-10/h1-9,14H,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human CA12 using CO2 as substrate incubated for 6 hrs prior to substrate addition measured for 10 to 100 secs by stopped flow technique |
Bioorg Med Chem Lett 22: 5801-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.094
BindingDB Entry DOI: 10.7270/Q2319X0G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50337278

(2-(phenylamino)benzoic acid | 2-Phenylamino-benzoi...)Show InChI InChI=1S/C13H11NO2/c15-13(16)11-8-4-5-9-12(11)14-10-6-2-1-3-7-10/h1-9,14H,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 using CO2 as substrate incubated for 6 hrs prior to substrate addition measured for 10 to 100 secs by stopped flow technique |
Bioorg Med Chem Lett 22: 5801-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.094
BindingDB Entry DOI: 10.7270/Q2319X0G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50337278

(2-(phenylamino)benzoic acid | 2-Phenylamino-benzoi...)Show InChI InChI=1S/C13H11NO2/c15-13(16)11-8-4-5-9-12(11)14-10-6-2-1-3-7-10/h1-9,14H,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using CO2 as substrate incubated for 6 hrs prior to substrate addition measured for 10 to 100 secs by stopped flow technique |
Bioorg Med Chem Lett 22: 5801-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.094
BindingDB Entry DOI: 10.7270/Q2319X0G |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein 4 (FABP4)
(Homo sapiens (Human)) | BDBM50337278

(2-(phenylamino)benzoic acid | 2-Phenylamino-benzoi...)Show InChI InChI=1S/C13H11NO2/c15-13(16)11-8-4-5-9-12(11)14-10-6-2-1-3-7-10/h1-9,14H,(H,15,16) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 5.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of 1,8-ANS from recombinant human 6His-tagged FABP4 expressed in Escherichia coli BL21 DE3 incubated for 15 mins followed by 1,8-ANS add... |
J Med Chem 63: 4090-4106 (2020)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50337278

(2-(phenylamino)benzoic acid | 2-Phenylamino-benzoi...)Show InChI InChI=1S/C13H11NO2/c15-13(16)11-8-4-5-9-12(11)14-10-6-2-1-3-7-10/h1-9,14H,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C2 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assay |
J Med Chem 55: 2311-23 (2012)
Article DOI: 10.1021/jm201547v
BindingDB Entry DOI: 10.7270/Q2C24XGP |
More data for this
Ligand-Target Pair | |
NAD-Dependent Deacetylase Sirtuin-1
(Homo sapiens (Human)) | BDBM50337278

(2-(phenylamino)benzoic acid | 2-Phenylamino-benzoi...)Show InChI InChI=1S/C13H11NO2/c15-13(16)11-8-4-5-9-12(11)14-10-6-2-1-3-7-10/h1-9,14H,(H,15,16) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant SIRT1 using Fluor de Lys-SIRT as substrate incubated for 60 mins prior to substrate addition measured after 60 mins b... |
J Med Chem 55: 5760-73 (2012)
Article DOI: 10.1021/jm3002108
BindingDB Entry DOI: 10.7270/Q2WW7JRS |
More data for this
Ligand-Target Pair | |
17-beta-Hydroxysteroid Dehydrogenase 5 (17-beta-HSD5, AKR1C3)
(Homo sapiens (Human)) | BDBM50337278

(2-(phenylamino)benzoic acid | 2-Phenylamino-benzoi...)Show InChI InChI=1S/C13H11NO2/c15-13(16)11-8-4-5-9-12(11)14-10-6-2-1-3-7-10/h1-9,14H,(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assay |
J Med Chem 55: 2311-23 (2012)
Article DOI: 10.1021/jm201547v
BindingDB Entry DOI: 10.7270/Q2C24XGP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
NAD-dependent deacetylase sirtuin 1
(Homo sapiens (Human)) | BDBM50337278

(2-(phenylamino)benzoic acid | 2-Phenylamino-benzoi...)Show InChI InChI=1S/C13H11NO2/c15-13(16)11-8-4-5-9-12(11)14-10-6-2-1-3-7-10/h1-9,14H,(H,15,16) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Jambheshwar University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of SIRT1 (unknown origin) using acetylated lysine as substrate by Fluor de Lys assay |
Eur J Med Chem 119: 45-69 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.063
BindingDB Entry DOI: 10.7270/Q2VH5QTW |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50337278

(2-(phenylamino)benzoic acid | 2-Phenylamino-benzoi...)Show InChI InChI=1S/C13H11NO2/c15-13(16)11-8-4-5-9-12(11)14-10-6-2-1-3-7-10/h1-9,14H,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C2 by fluorimetric method |
Bioorg Med Chem Lett 21: 1464-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.010
BindingDB Entry DOI: 10.7270/Q24J0FD4 |
More data for this
Ligand-Target Pair | |
17-beta-Hydroxysteroid Dehydrogenase 5 (17-beta-HSD5, AKR1C3)
(Homo sapiens (Human)) | BDBM50337278

(2-(phenylamino)benzoic acid | 2-Phenylamino-benzoi...)Show InChI InChI=1S/C13H11NO2/c15-13(16)11-8-4-5-9-12(11)14-10-6-2-1-3-7-10/h1-9,14H,(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 by fluorimetric method |
Bioorg Med Chem Lett 21: 1464-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.010
BindingDB Entry DOI: 10.7270/Q24J0FD4 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50337278

(2-(phenylamino)benzoic acid | 2-Phenylamino-benzoi...)Show InChI InChI=1S/C13H11NO2/c15-13(16)11-8-4-5-9-12(11)14-10-6-2-1-3-7-10/h1-9,14H,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | |
17-beta-Hydroxysteroid Dehydrogenase 5 (17-beta-HSD5, AKR1C3)
(Homo sapiens (Human)) | BDBM50337278

(2-(phenylamino)benzoic acid | 2-Phenylamino-benzoi...)Show InChI InChI=1S/C13H11NO2/c15-13(16)11-8-4-5-9-12(11)14-10-6-2-1-3-7-10/h1-9,14H,(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
NAD-dependent deacetylase sirtuin 2
(Homo sapiens (Human)) | BDBM50337278

(2-(phenylamino)benzoic acid | 2-Phenylamino-benzoi...)Show InChI InChI=1S/C13H11NO2/c15-13(16)11-8-4-5-9-12(11)14-10-6-2-1-3-7-10/h1-9,14H,(H,15,16) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant SIRT2 using Fluor de Lys-SIRT as substrate incubated for 60 mins prior to substrate addition measured after 60 mins b... |
J Med Chem 55: 5760-73 (2012)
Article DOI: 10.1021/jm3002108
BindingDB Entry DOI: 10.7270/Q2WW7JRS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data