

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50341335 CHEMBL1766474::rel-(1R,2S,4R,5S)-6-[[1-(4-Cyclohexylbutyl)-1H-1,2,3-triazol-4-yl]methylamino]cyclohexane-1,2,3,4,5-pentaol
SMILES: O[C@H]1[C@H](O)[C@@H](O)[C@H](NCc2cn(CCCCC3CCCCC3)nn2)[C@@H](O)[C@@H]1O
InChI Key: InChIKey=SCLOQUUDNGPMRM-OXHPVDPFSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50341335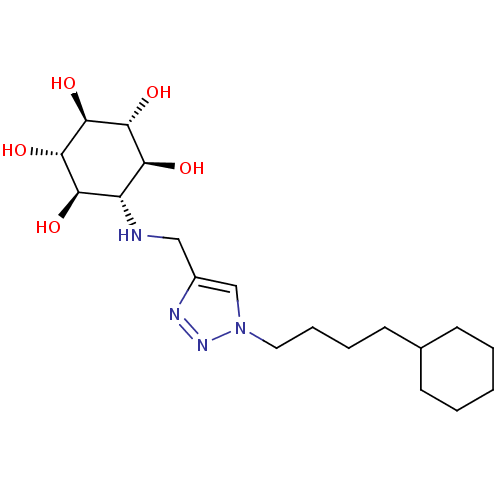 (CHEMBL1766474 | rel-(1R,2S,4R,5S)-6-[[1-(4-Cyclohe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Competitive inhibition of recombinant glucocerebrosidase | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| α-glucosidase (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50341335 (CHEMBL1766474 | rel-(1R,2S,4R,5S)-6-[[1-(4-Cyclohe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50341335 (CHEMBL1766474 | rel-(1R,2S,4R,5S)-6-[[1-(4-Cyclohe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Inhibition of recombinant glucocerebrosidase in McIlvaine buffer at pH 5.2 after 10 mins by fluorometric analysis | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| α-galactosidase (Coffea arabica (Coffee beans)) | BDBM50341335 (CHEMBL1766474 | rel-(1R,2S,4R,5S)-6-[[1-(4-Cyclohe...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Inhibition of alpha-galactosidase from coffee beans | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| β-galactosidase (Bos taurus (Bovine)) | BDBM50341335 (CHEMBL1766474 | rel-(1R,2S,4R,5S)-6-[[1-(4-Cyclohe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50341335 (CHEMBL1766474 | rel-(1R,2S,4R,5S)-6-[[1-(4-Cyclohe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Inhibition of recombinant glucocerebrosidase in McIlvaine buffer at pH 7.4 after 10 mins by fluorometric analysis | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable alpha-glucosidase Os06g0675700 (Oryza sativa subsp. japonica) | BDBM50341335 (CHEMBL1766474 | rel-(1R,2S,4R,5S)-6-[[1-(4-Cyclohe...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Inhibition of rice alpha-glucosidase | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||