

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50341410 CHEMBL4166144
SMILES: COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC2CCOCC2)c2nc(N)nc(C)c2c1
InChI Key: InChIKey=FEHPMLVVSQNYOF-UHFFFAOYSA-N
Data: 14 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50341410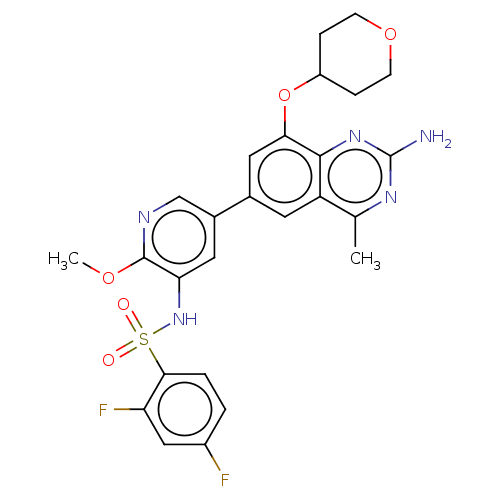 (CHEMBL4166144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes assessed as reduction in 6-Hydroxychlorzoxazone formation using chlorzoxazone as substrate after 10 to... | J Med Chem 61: 6087-6109 (2018) Article DOI: 10.1021/acs.jmedchem.8b00416 BindingDB Entry DOI: 10.7270/Q28K7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50341410 (CHEMBL4166144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate by ADP-Glo assay | J Med Chem 61: 6087-6109 (2018) Article DOI: 10.1021/acs.jmedchem.8b00416 BindingDB Entry DOI: 10.7270/Q28K7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50341410 (CHEMBL4166144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay | J Med Chem 61: 6087-6109 (2018) Article DOI: 10.1021/acs.jmedchem.8b00416 BindingDB Entry DOI: 10.7270/Q28K7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50341410 (CHEMBL4166144) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of AKT1 (unknown origin) using FAM-labeled peptide as substrate by mobility shift assay | J Med Chem 61: 6087-6109 (2018) Article DOI: 10.1021/acs.jmedchem.8b00416 BindingDB Entry DOI: 10.7270/Q28K7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50341410 (CHEMBL4166144) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes assessed as reduction in acetaminophen formation using phenacetin as substrate after 10 to 20 mins in ... | J Med Chem 61: 6087-6109 (2018) Article DOI: 10.1021/acs.jmedchem.8b00416 BindingDB Entry DOI: 10.7270/Q28K7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50341410 (CHEMBL4166144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate by ADP-Glo assay | J Med Chem 61: 6087-6109 (2018) Article DOI: 10.1021/acs.jmedchem.8b00416 BindingDB Entry DOI: 10.7270/Q28K7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| p110α/p85α (Homo sapiens (Human)) | BDBM50341410 (CHEMBL4166144) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal FLAG-tagged full-length p110alpha/human p85alpha expressed in Sf9 insect cells using PIP2 as substrate by ... | J Med Chem 62: 8873-8879 (2019) Article DOI: 10.1021/acs.jmedchem.9b00969 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50341410 (CHEMBL4166144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of mTOR (unknown origin) using ULight-4E-BP1 peptide as substrate after 30 mins by Lance Ultra assay | J Med Chem 61: 6087-6109 (2018) Article DOI: 10.1021/acs.jmedchem.8b00416 BindingDB Entry DOI: 10.7270/Q28K7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50341410 (CHEMBL4166144) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cell membranes by patch clamp method | J Med Chem 61: 6087-6109 (2018) Article DOI: 10.1021/acs.jmedchem.8b00416 BindingDB Entry DOI: 10.7270/Q28K7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50341410 (CHEMBL4166144) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes assessed as reduction in dextrophan formation using detromethorphann as substrate after 10 to 20 mins ... | J Med Chem 61: 6087-6109 (2018) Article DOI: 10.1021/acs.jmedchem.8b00416 BindingDB Entry DOI: 10.7270/Q28K7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50341410 (CHEMBL4166144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes assessed as reduction in 4-Hydroxydiclofenac formation using diclofenac as substrate after 10 to 20 mi... | J Med Chem 61: 6087-6109 (2018) Article DOI: 10.1021/acs.jmedchem.8b00416 BindingDB Entry DOI: 10.7270/Q28K7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50341410 (CHEMBL4166144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as reduction in 1-Hydroxymidazolam formation using midazolam as substrate after 10 to 20 mins... | J Med Chem 61: 6087-6109 (2018) Article DOI: 10.1021/acs.jmedchem.8b00416 BindingDB Entry DOI: 10.7270/Q28K7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50341410 (CHEMBL4166144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes assessed as reduction in 4-Hydroxymephenytoinn formation using mephenytoin as substrate after 10 to 2... | J Med Chem 61: 6087-6109 (2018) Article DOI: 10.1021/acs.jmedchem.8b00416 BindingDB Entry DOI: 10.7270/Q28K7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50341410 (CHEMBL4166144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate by ADP-Glo assay | J Med Chem 61: 6087-6109 (2018) Article DOI: 10.1021/acs.jmedchem.8b00416 BindingDB Entry DOI: 10.7270/Q28K7CNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||