

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM5035 CHEMBL235747::N-(5-tert-Butyl-3,4-dimethyl-1,3-thiazol-2(3H)-ylidene)benzenesulfonamide::N-[(2Z)-5-tert-butyl-3,4-dimethyl-2,3-dihydro-1,3-thiazol-2-ylidene]benzenesulfonamide::thiazolidenebenzenesulfonamide deriv. 10r
SMILES: Cc1c(s\c(=N/S(=O)(=O)c2ccccc2)n1C)C(C)(C)C
InChI Key: InChIKey=XYRMLZVYRVONRR-PEZBUJJGSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Reverse Transcriptase (Human immunodeficiency virus type 1) | BDBM5035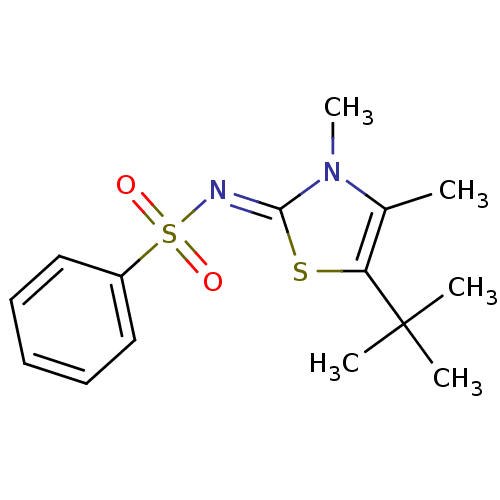 (CHEMBL235747 | N-(5-tert-Butyl-3,4-dimethyl-1,3-th...) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 Reverse Transcriptase Mutant (K103N) (Human immunodeficiency virus type 1) | BDBM5035 (CHEMBL235747 | N-(5-tert-Butyl-3,4-dimethyl-1,3-th...) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM5035 (CHEMBL235747 | N-(5-tert-Butyl-3,4-dimethyl-1,3-th...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H] CP-55,940 from human CB2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5133-5 (2007) Article DOI: 10.1016/j.bmcl.2007.07.005 BindingDB Entry DOI: 10.7270/Q27944C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM5035 (CHEMBL235747 | N-(5-tert-Butyl-3,4-dimethyl-1,3-th...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells by [35S]GTPgamaS binding assay | Bioorg Med Chem Lett 17: 5133-5 (2007) Article DOI: 10.1016/j.bmcl.2007.07.005 BindingDB Entry DOI: 10.7270/Q27944C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM5035 (CHEMBL235747 | N-(5-tert-Butyl-3,4-dimethyl-1,3-th...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H] CP-55,940 from human CB1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5133-5 (2007) Article DOI: 10.1016/j.bmcl.2007.07.005 BindingDB Entry DOI: 10.7270/Q27944C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 Reverse Transcriptase Mutant (Y181C) (Human immunodeficiency virus type 1) | BDBM5035 (CHEMBL235747 | N-(5-tert-Butyl-3,4-dimethyl-1,3-th...) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||