

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50358212 CHEMBL1922218::US8846696, (2S3R)-3-{9-Isopropyl-6-[(pyridin-3-ylmethyl)-amino]-9H-purin-2-ylamino}-pentan-2-ol
SMILES: CC[C@@H](Nc1nc(NCc2cccnc2)c2ncn(C(C)C)c2n1)[C@H](C)O
InChI Key: InChIKey=SCACHXWSWJBIHG-DZGCQCFKSA-N
Data: 10 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50358212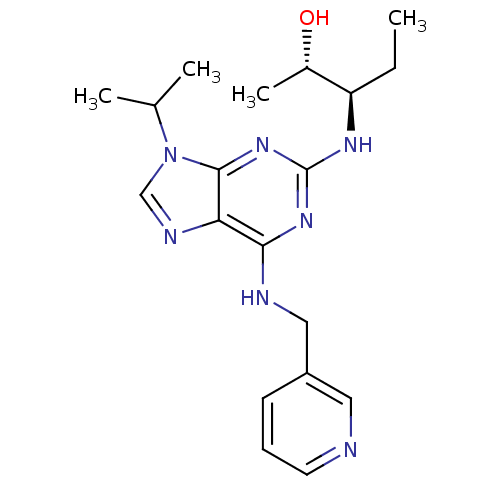 (CHEMBL1922218 | US8846696, (2S3R)-3-{9-Isopropyl-6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cyclacel Limited; Cancer Research Technology Limited US Patent | Assay Description The compounds from the examples below were investigated for their CDK2/cyclin E, CDK1/cyclin B, CDK4/cyclin D1 and CDK7/cyclin H, ERK-2, and PKA inhi... | US Patent US8846696 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM50358212 (CHEMBL1922218 | US8846696, (2S3R)-3-{9-Isopropyl-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cyclacel Limited; Cancer Research Technology Limited US Patent | Assay Description The compounds from the examples below were investigated for their CDK2/cyclin E, CDK1/cyclin B, CDK4/cyclin D1 and CDK7/cyclin H, ERK-2, and PKA inhi... | US Patent US8846696 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM50358212 (CHEMBL1922218 | US8846696, (2S3R)-3-{9-Isopropyl-6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cyclacel Limited; Cancer Research Technology Limited US Patent | Assay Description The compounds from the examples below were investigated for their CDK2/cyclin E, CDK1/cyclin B, CDK4/cyclin D1 and CDK7/cyclin H, ERK-2, and PKA inhi... | US Patent US8846696 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM50358212 (CHEMBL1922218 | US8846696, (2S3R)-3-{9-Isopropyl-6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cyclacel Limited; Cancer Research Technology Limited US Patent | Assay Description The compounds from the examples below were investigated for their CDK2/cyclin E, CDK1/cyclin B, CDK4/cyclin D1 and CDK7/cyclin H, ERK-2, and PKA inhi... | US Patent US8846696 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Dependent Kinase 1 (CDK1) (Homo sapiens (Human)) | BDBM50358212 (CHEMBL1922218 | US8846696, (2S3R)-3-{9-Isopropyl-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of CDK1/cyclin B | Bioorg Med Chem 19: 6949-65 (2011) Article DOI: 10.1016/j.bmc.2011.08.051 BindingDB Entry DOI: 10.7270/Q2NP24V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50358212 (CHEMBL1922218 | US8846696, (2S3R)-3-{9-Isopropyl-6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cyclacel Limited; Cancer Research Technology Limited US Patent | Assay Description The compounds from the examples below were investigated for their CDK2/cyclin E, CDK1/cyclin B, CDK4/cyclin D1 and CDK7/cyclin H, ERK-2, and PKA inhi... | US Patent US8846696 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Dependent Kinase 9 (CDK9) (Homo sapiens (Human)) | BDBM50358212 (CHEMBL1922218 | US8846696, (2S3R)-3-{9-Isopropyl-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of CDK9/cyclin T1 | Bioorg Med Chem 19: 6949-65 (2011) Article DOI: 10.1016/j.bmc.2011.08.051 BindingDB Entry DOI: 10.7270/Q2NP24V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Dependent Kinase 7 (CDK7) (Homo sapiens (Human)) | BDBM50358212 (CHEMBL1922218 | US8846696, (2S3R)-3-{9-Isopropyl-6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of CDK7/cyclin H | Bioorg Med Chem 19: 6949-65 (2011) Article DOI: 10.1016/j.bmc.2011.08.051 BindingDB Entry DOI: 10.7270/Q2NP24V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50358212 (CHEMBL1922218 | US8846696, (2S3R)-3-{9-Isopropyl-6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of ERK2 | Bioorg Med Chem 19: 6949-65 (2011) Article DOI: 10.1016/j.bmc.2011.08.051 BindingDB Entry DOI: 10.7270/Q2NP24V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase (PKA) (Homo sapiens (Human)) | BDBM50358212 (CHEMBL1922218 | US8846696, (2S3R)-3-{9-Isopropyl-6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cyclacel Limited; Cancer Research Technology Limited US Patent | Assay Description The compounds from the examples below were investigated for their CDK2/cyclin E, CDK1/cyclin B, CDK4/cyclin D1 and CDK7/cyclin H, ERK-2, and PKA inhi... | US Patent US8846696 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||