

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50359899 CHEMBL263909
SMILES: Clc1ccc(\C=C2/S\C(NC2=O)=N/c2nsc3ccccc23)cc1
InChI Key: InChIKey=PUFLXTPEEIWEIM-ZROIWOOFSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50359899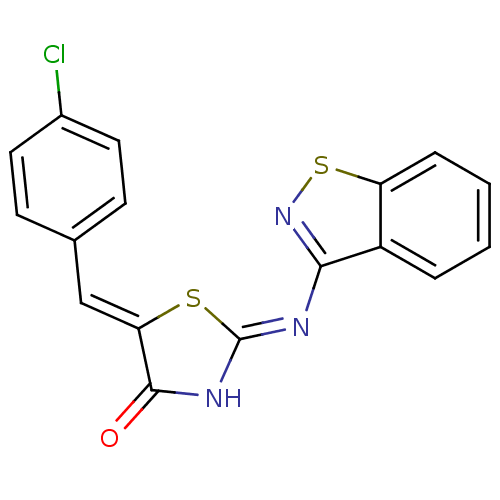 (CHEMBL263909) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Inhibition of MMP3 catalytic domain (unknown origin) assessed as reduction in hydrolysis of Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate measured fo... | Bioorg Med Chem 23: 1551-6 (2015) Article DOI: 10.1016/j.bmc.2015.02.002 BindingDB Entry DOI: 10.7270/Q26T0P92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase-1 (Glycine max (soybean)) | BDBM50359899 (CHEMBL263909) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexander Technological Education Institute of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of soybean LOX type 1 pre-incubated for 4 mins at 7x10'-7 w/v concentration assessed as inhibition of sodium linoleate to 13-hydroperoxyli... | Eur J Med Chem 47: 111-24 (2012) Article DOI: 10.1016/j.ejmech.2011.10.029 BindingDB Entry DOI: 10.7270/Q2K937ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase (cyclooxygenase) (Ovis aries (Sheep)) | BDBM50359899 (CHEMBL263909) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexander Technological Education Institute of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of ovine COX1 assessed as PGF2alpha production by enzyme immunoassay | Eur J Med Chem 47: 111-24 (2012) Article DOI: 10.1016/j.ejmech.2011.10.029 BindingDB Entry DOI: 10.7270/Q2K937ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50359899 (CHEMBL263909) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Inhibition of MMP13 catalytic domain (unknown origin) assessed as reduction in hydrolysis of Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate measured f... | Bioorg Med Chem 23: 1551-6 (2015) Article DOI: 10.1016/j.bmc.2015.02.002 BindingDB Entry DOI: 10.7270/Q26T0P92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||