

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50363489 CHEMBL1946179::hCA inhibitor, 9
SMILES: COc1c(Br)cc(Br)c(Br)c1OC
InChI Key: InChIKey=RLQZRYRMTTWFCU-UHFFFAOYSA-N
Data: 8 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50363489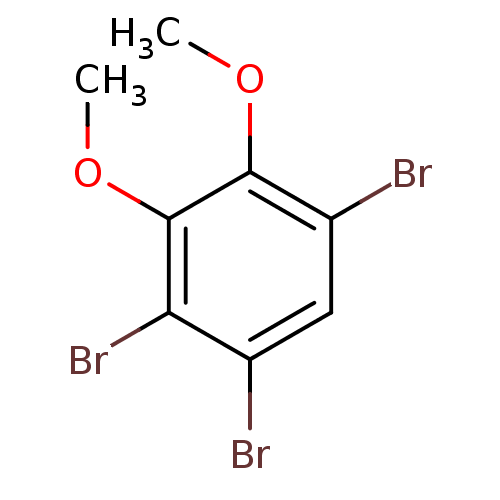 (CHEMBL1946179 | hCA inhibitor, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human CA4 using 4NPA as substrate for 3 mins by Lineweaver burk plot analysis | Eur J Med Chem 54: 423-8 (2012) Article DOI: 10.1016/j.ejmech.2012.05.025 BindingDB Entry DOI: 10.7270/Q2WS8V91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50363489 (CHEMBL1946179 | hCA inhibitor, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human CA2 using 4NPA as substrate for 3 mins by Lineweaver burk plot analysis | Eur J Med Chem 54: 423-8 (2012) Article DOI: 10.1016/j.ejmech.2012.05.025 BindingDB Entry DOI: 10.7270/Q2WS8V91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50363489 (CHEMBL1946179 | hCA inhibitor, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Artvin£oruh University Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 esterase activity using 4-nitrophenylacetate as substrate | Bioorg Med Chem Lett 22: 1352-7 (2012) Article DOI: 10.1016/j.bmcl.2011.12.069 BindingDB Entry DOI: 10.7270/Q2WH2QF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50363489 (CHEMBL1946179 | hCA inhibitor, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Artvin£oruh University Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 4 esterase activity using 4-nitrophenylacetate as substrate | Bioorg Med Chem Lett 22: 1352-7 (2012) Article DOI: 10.1016/j.bmcl.2011.12.069 BindingDB Entry DOI: 10.7270/Q2WH2QF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50363489 (CHEMBL1946179 | hCA inhibitor, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Artvin£oruh University Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 1 esterase activity using 4-nitrophenylacetate as substrate | Bioorg Med Chem Lett 22: 1352-7 (2012) Article DOI: 10.1016/j.bmcl.2011.12.069 BindingDB Entry DOI: 10.7270/Q2WH2QF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50363489 (CHEMBL1946179 | hCA inhibitor, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Artvin Coruh University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 27: 467-75 (2012) Article DOI: 10.3109/14756366.2011.596836 BindingDB Entry DOI: 10.7270/Q2CN72TT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (CA-VI) (Homo sapiens (Human)) | BDBM50363489 (CHEMBL1946179 | hCA inhibitor, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Artvin Coruh University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 27: 467-75 (2012) Article DOI: 10.3109/14756366.2011.596836 BindingDB Entry DOI: 10.7270/Q2CN72TT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (CA-VI) (Homo sapiens (Human)) | BDBM50363489 (CHEMBL1946179 | hCA inhibitor, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Artvin£oruh University Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 6 esterase activity using 4-nitrophenylacetate as substrate | Bioorg Med Chem Lett 22: 1352-7 (2012) Article DOI: 10.1016/j.bmcl.2011.12.069 BindingDB Entry DOI: 10.7270/Q2WH2QF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||