 Found 11 hits for monomerid = 50363494
Found 11 hits for monomerid = 50363494 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 6 (CA-VI)
(Homo sapiens (Human)) | BDBM50363494
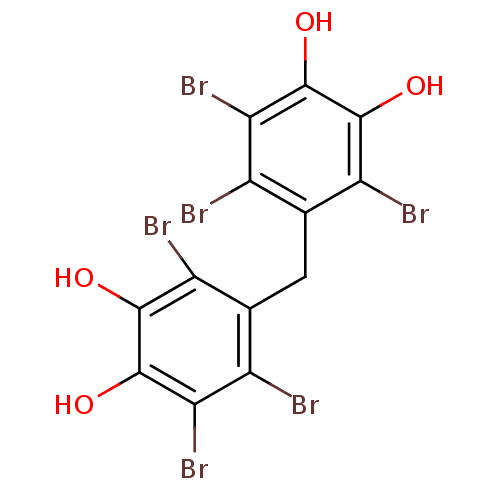
(5,5'-methylenebis(3,4,6-tribromo-benzene-1,2-d...)Show SMILES Oc1c(O)c(Br)c(Cc2c(Br)c(O)c(O)c(Br)c2Br)c(Br)c1Br Show InChI InChI=1S/C13H6Br6O4/c14-4-2(6(16)10(20)12(22)8(4)18)1-3-5(15)9(19)13(23)11(21)7(3)17/h20-23H,1H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Artvin£oruh University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 6 esterase activity using 4-nitrophenylacetate as substrate |
Bioorg Med Chem Lett 22: 1352-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.069
BindingDB Entry DOI: 10.7270/Q2WH2QF4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50363494

(5,5'-methylenebis(3,4,6-tribromo-benzene-1,2-d...)Show SMILES Oc1c(O)c(Br)c(Cc2c(Br)c(O)c(O)c(Br)c2Br)c(Br)c1Br Show InChI InChI=1S/C13H6Br6O4/c14-4-2(6(16)10(20)12(22)8(4)18)1-3-5(15)9(19)13(23)11(21)7(3)17/h20-23H,1H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Artvin£oruh University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 esterase activity using 4-nitrophenylacetate as substrate |
Bioorg Med Chem Lett 22: 1352-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.069
BindingDB Entry DOI: 10.7270/Q2WH2QF4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50363494

(5,5'-methylenebis(3,4,6-tribromo-benzene-1,2-d...)Show SMILES Oc1c(O)c(Br)c(Cc2c(Br)c(O)c(O)c(Br)c2Br)c(Br)c1Br Show InChI InChI=1S/C13H6Br6O4/c14-4-2(6(16)10(20)12(22)8(4)18)1-3-5(15)9(19)13(23)11(21)7(3)17/h20-23H,1H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Artvin£oruh University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 4 esterase activity using 4-nitrophenylacetate as substrate |
Bioorg Med Chem Lett 22: 1352-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.069
BindingDB Entry DOI: 10.7270/Q2WH2QF4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50363494

(5,5'-methylenebis(3,4,6-tribromo-benzene-1,2-d...)Show SMILES Oc1c(O)c(Br)c(Cc2c(Br)c(O)c(O)c(Br)c2Br)c(Br)c1Br Show InChI InChI=1S/C13H6Br6O4/c14-4-2(6(16)10(20)12(22)8(4)18)1-3-5(15)9(19)13(23)11(21)7(3)17/h20-23H,1H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Artvin£oruh University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 esterase activity using 4-nitrophenylacetate as substrate |
Bioorg Med Chem Lett 22: 1352-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.069
BindingDB Entry DOI: 10.7270/Q2WH2QF4 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1C
(Homo sapiens (Human)) | BDBM50363494

(5,5'-methylenebis(3,4,6-tribromo-benzene-1,2-d...)Show SMILES Oc1c(O)c(Br)c(Cc2c(Br)c(O)c(O)c(Br)c2Br)c(Br)c1Br Show InChI InChI=1S/C13H6Br6O4/c14-4-2(6(16)10(20)12(22)8(4)18)1-3-5(15)9(19)13(23)11(21)7(3)17/h20-23H,1H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SHP1 using OMFP as substrate after 3 mins |
Bioorg Med Chem Lett 22: 2827-32 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.074
BindingDB Entry DOI: 10.7270/Q28K7B33 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase F (LAR)
(Homo sapiens (Human)) | BDBM50363494

(5,5'-methylenebis(3,4,6-tribromo-benzene-1,2-d...)Show SMILES Oc1c(O)c(Br)c(Cc2c(Br)c(O)c(O)c(Br)c2Br)c(Br)c1Br Show InChI InChI=1S/C13H6Br6O4/c14-4-2(6(16)10(20)12(22)8(4)18)1-3-5(15)9(19)13(23)11(21)7(3)17/h20-23H,1H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LAR using OMFP as substrate after 3 mins |
Bioorg Med Chem Lett 22: 2827-32 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.074
BindingDB Entry DOI: 10.7270/Q28K7B33 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50363494

(5,5'-methylenebis(3,4,6-tribromo-benzene-1,2-d...)Show SMILES Oc1c(O)c(Br)c(Cc2c(Br)c(O)c(O)c(Br)c2Br)c(Br)c1Br Show InChI InChI=1S/C13H6Br6O4/c14-4-2(6(16)10(20)12(22)8(4)18)1-3-5(15)9(19)13(23)11(21)7(3)17/h20-23H,1H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SHP2 using OMFP as substrate after 3 mins |
Bioorg Med Chem Lett 22: 2827-32 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.074
BindingDB Entry DOI: 10.7270/Q28K7B33 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50363494

(5,5'-methylenebis(3,4,6-tribromo-benzene-1,2-d...)Show SMILES Oc1c(O)c(Br)c(Cc2c(Br)c(O)c(O)c(Br)c2Br)c(Br)c1Br Show InChI InChI=1S/C13H6Br6O4/c14-4-2(6(16)10(20)12(22)8(4)18)1-3-5(15)9(19)13(23)11(21)7(3)17/h20-23H,1H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP after 3 mins |
Bioorg Med Chem Lett 22: 2827-32 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.074
BindingDB Entry DOI: 10.7270/Q28K7B33 |
More data for this
Ligand-Target Pair | |
Aldose reductase
(Homo sapiens (Human)) | BDBM50363494

(5,5'-methylenebis(3,4,6-tribromo-benzene-1,2-d...)Show SMILES Oc1c(O)c(Br)c(Cc2c(Br)c(O)c(O)c(Br)c2Br)c(Br)c1Br Show InChI InChI=1S/C13H6Br6O4/c14-4-2(6(16)10(20)12(22)8(4)18)1-3-5(15)9(19)13(23)11(21)7(3)17/h20-23H,1H2 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 566 | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin Province Academy of Traditional Chinese Medicine and Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of human muscle recombinant aldose reductase by spectrophotometry |
J Nat Prod 68: 620-2 (2005)
Article DOI: 10.1021/np040199j
BindingDB Entry DOI: 10.7270/Q2BZ68TQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50363494

(5,5'-methylenebis(3,4,6-tribromo-benzene-1,2-d...)Show SMILES Oc1c(O)c(Br)c(Cc2c(Br)c(O)c(O)c(Br)c2Br)c(Br)c1Br Show InChI InChI=1S/C13H6Br6O4/c14-4-2(6(16)10(20)12(22)8(4)18)1-3-5(15)9(19)13(23)11(21)7(3)17/h20-23H,1H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Atatürk University
| Assay Description
Carbonic anhydrase activity was assayed by following the hydration of CO2 according to our previous studies [Soyut et al., Protein Pept. Lett., 15:52... |
J Enzyme Inhib Med Chem 27: 43-50 (2012)
Article DOI: 10.3109/14756366.2011.574131
BindingDB Entry DOI: 10.7270/Q2CR5S75 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50363494

(5,5'-methylenebis(3,4,6-tribromo-benzene-1,2-d...)Show SMILES Oc1c(O)c(Br)c(Cc2c(Br)c(O)c(O)c(Br)c2Br)c(Br)c1Br Show InChI InChI=1S/C13H6Br6O4/c14-4-2(6(16)10(20)12(22)8(4)18)1-3-5(15)9(19)13(23)11(21)7(3)17/h20-23H,1H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human recombinant PTB1B using pNPP as substrate after 3 mins |
Bioorg Med Chem Lett 22: 2827-32 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.074
BindingDB Entry DOI: 10.7270/Q28K7B33 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data