

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50369775 Mavik::RU-44570::TRANDOLAPRIL
SMILES: CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1[C@H]2CCCC[C@@H]2C[C@H]1C(O)=O
InChI Key: InChIKey=VXFJYXUZANRPDJ-WTNASJBWSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50369775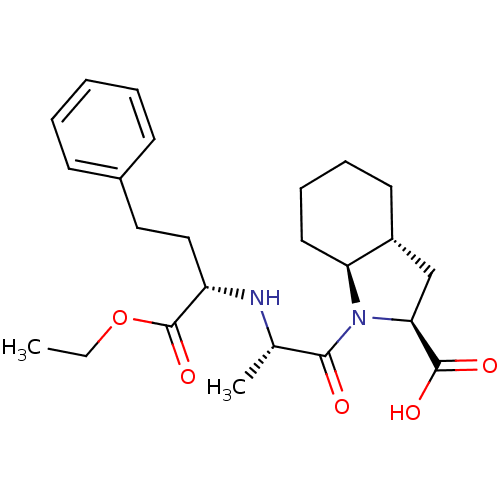 (Mavik | RU-44570 | TRANDOLAPRIL) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | DrugBank PubMed | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Inhibitory activity against angiotensin I converting enzyme (ACE) | J Med Chem 43: 305-41 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q2JD4XH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 3 (Homo sapiens (Human)) | BDBM50369775 (Mavik | RU-44570 | TRANDOLAPRIL) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM50369775 (Mavik | RU-44570 | TRANDOLAPRIL) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 4 (Homo sapiens (Human)) | BDBM50369775 (Mavik | RU-44570 | TRANDOLAPRIL) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||