

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50370754 CHEMBL252929
SMILES: Nc1nc2N(CNc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O
InChI Key: InChIKey=KITLPLLNIZOYIJ-UUOKFMHZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-(1,3)-fucosyltransferase 9 (Homo sapiens (Human)) | BDBM50370754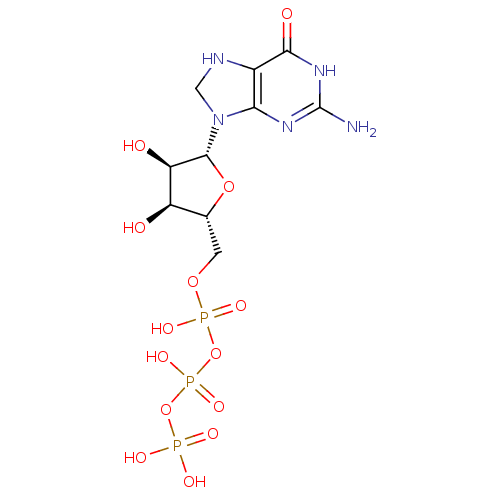 (CHEMBL252929) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hamburg University Curated by ChEMBL | Assay Description Inhibition of human recombinant alpha1,3-fucosyltransferase 9 using GDP-[14C]-fucose preincubated for 30 mins by liquid scintillation counting | Bioorg Med Chem 22: 6430-7 (2014) Article DOI: 10.1016/j.bmc.2014.09.038 BindingDB Entry DOI: 10.7270/Q2G73GC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketopantoate reductase (Escherichia coli (strain K12)) | BDBM50370754 (CHEMBL252929) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.53E+5 | n/a | n/a | n/a | n/a | n/a |
University Chemical Laboratory Curated by ChEMBL | Assay Description Binding affinity to Escherichia coli KPR | J Med Chem 49: 4992-5000 (2006) Article DOI: 10.1021/jm060490r BindingDB Entry DOI: 10.7270/Q28S4QQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-(1,3)-fucosyltransferase 9 (Homo sapiens (Human)) | BDBM50370754 (CHEMBL252929) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamburg University Curated by ChEMBL | Assay Description Inhibition of human recombinant alpha1,3-fucosyltransferase 9 using GDP-[14C]-fucose preincubated for 30 mins by liquid scintillation counting | Bioorg Med Chem 22: 6430-7 (2014) Article DOI: 10.1016/j.bmc.2014.09.038 BindingDB Entry DOI: 10.7270/Q2G73GC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||