 Found 16 hits for monomerid = 50378739
Found 16 hits for monomerid = 50378739 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein-beta-aspartate methyltransferase
(Homo sapiens (Human)) | BDBM50378739
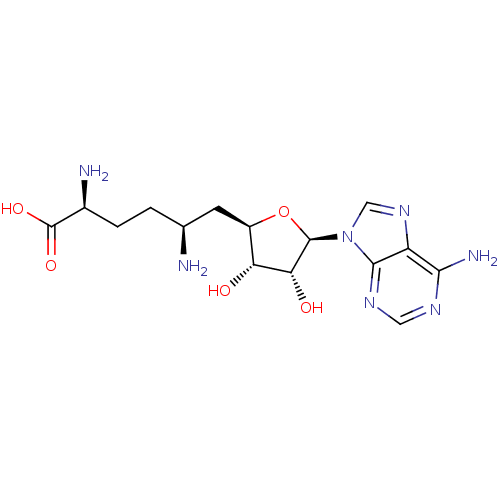
(SINEFUNGIN)Show SMILES N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
C.N.R.S.
Curated by ChEMBL
| Assay Description
Kinetic constant was measured for Protein Methylase II of Leishmania donovani promastigotes using supernatant (S12) fraction |
J Med Chem 35: 63-7 (1992)
BindingDB Entry DOI: 10.7270/Q2T1548J |
More data for this
Ligand-Target Pair | |
Protein-beta-aspartate methyltransferase
(Homo sapiens (Human)) | BDBM50378739

(SINEFUNGIN)Show SMILES N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 1.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
C.N.R.S.
Curated by ChEMBL
| Assay Description
Kinetic constant was measured for Protein Methylase II of Leishmania donovani promastigotes using protein extracted from the 12000g pellet(P12). |
J Med Chem 35: 63-7 (1992)
BindingDB Entry DOI: 10.7270/Q2T1548J |
More data for this
Ligand-Target Pair | |
Thiopurine S-methyltransferase
(Homo sapiens (Human)) | BDBM50378739

(SINEFUNGIN)Show SMILES N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Research and Development, LLC
Curated by ChEMBL
| Assay Description
Binding affinity to thiopurine methyltransferase (unknown origin) by NMR analysis |
J Med Chem 57: 7819-37 (2014)
Article DOI: 10.1021/jm500325k
BindingDB Entry DOI: 10.7270/Q2JW8GH3 |
More data for this
Ligand-Target Pair | |
SET domain-containing protein 7/9 (Set7/9)
(Homo sapiens (Human)) | BDBM50378739

(SINEFUNGIN)Show SMILES N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant SET7 after 90 mins by SDS-PAGE based scintillation counting |
Bioorg Med Chem Lett 20: 2103-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.069
BindingDB Entry DOI: 10.7270/Q2WQ04SW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein G9a (G9a)
(Homo sapiens (Human)) | BDBM50378739

(SINEFUNGIN)Show SMILES N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of EHMT2 (unknown origin) |
Bioorg Med Chem 25: 4579-4594 (2017)
Article DOI: 10.1016/j.bmc.2017.06.032
BindingDB Entry DOI: 10.7270/Q2CC135P |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT1
(Homo sapiens (Human)) | BDBM50378739

(SINEFUNGIN)Show SMILES N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of EHMT1 (unknown origin) |
Bioorg Med Chem 25: 4579-4594 (2017)
Article DOI: 10.1016/j.bmc.2017.06.032
BindingDB Entry DOI: 10.7270/Q2CC135P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50378739

(SINEFUNGIN)Show SMILES N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-SUMO tagged DOT1L (1 to 416 residues) expressed in Escherichia coli BL21(DE3) using oligonucleosome as substrate... |
Bioorg Med Chem 26: 1751-1758 (2018)
Article DOI: 10.1016/j.bmc.2018.02.020
BindingDB Entry DOI: 10.7270/Q2319ZHM |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT1/EHMT2
(Homo sapiens (Human)) | BDBM50378739

(SINEFUNGIN)Show SMILES N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of EHMT1 (unknown origin) preincubated for 5 mins followed by SAM and biotinylated H3K9 peptide addition measured after 120 mins by HTRF a... |
Bioorg Med Chem 25: 4579-4594 (2017)
Article DOI: 10.1016/j.bmc.2017.06.032
BindingDB Entry DOI: 10.7270/Q2CC135P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT1/EHMT2
(Homo sapiens (Human)) | BDBM50378739

(SINEFUNGIN)Show SMILES N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of EHMT2 (unknown origin) preincubated for 15 mins followed by SAM and biotinylated H3K9 peptide addition measured after 30 mins by TR-FRE... |
Bioorg Med Chem 25: 4579-4594 (2017)
Article DOI: 10.1016/j.bmc.2017.06.032
BindingDB Entry DOI: 10.7270/Q2CC135P |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase (NNMT)
(Homo sapiens (Human)) | BDBM50378739

(SINEFUNGIN)Show SMILES N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human full length NNMT expressed in Escherichia coli BL21(DE3) cells assessed as reduction in 1-methyl-nicotinamide formation... |
J Med Chem 62: 6597-6614 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00413 |
More data for this
Ligand-Target Pair | |
NS5
(Zika virus) | BDBM50378739

(SINEFUNGIN)Show SMILES N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Zika virus MTase (4 to 278 residues) expressed in Escherichia coli T7 using GpppAC4 as substrate in presence of [3H]SAM incubated for 3... |
J Med Chem 63: 470-489 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00775 |
More data for this
Ligand-Target Pair | |
NS5
(Zika virus) | BDBM50378739

(SINEFUNGIN)Show SMILES N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Zika virus MTase (4 to 278 residues) expressed in Escherichia coli T7 using A27 RNA as substrate in presence of [3H]SAM incubated for 3... |
J Med Chem 63: 470-489 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00775 |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50378739

(SINEFUNGIN)Show SMILES N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan
Curated by ChEMBL
| Assay Description
Inhibition of full-length N-terminal FLAG-tagged PRMT5 (unknown origin) (1 to 637 residues) expressed in baculovirus infected Sf9 insect cells using ... |
Bioorg Med Chem Lett 28: 3693-3699 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.026 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nonstructural protein 5
(Dengue virus) | BDBM50378739

(SINEFUNGIN)Show SMILES N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University
Curated by ChEMBL
| Assay Description
Inhibition of Dengue virus ribose 2'-O methyltransferase using RNA substrate after 20 mins in presence of [methyl-3H]-AdoMet by microbeta counting an... |
J Med Chem 59: 5622-49 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01653 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT1
(Homo sapiens (Human)) | BDBM50378739

(SINEFUNGIN)Show SMILES N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of EHMT1 (unknown origin)-mediated dimethylation of a biotinylated histone H3 peptide at lysine-9 by FRET-ba... |
ACS Med Chem Lett 5: 293-7 (2014)
Article DOI: 10.1021/ml4002503
BindingDB Entry DOI: 10.7270/Q2N58NWS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein G9a (G9a)
(Homo sapiens (Human)) | BDBM50378739

(SINEFUNGIN)Show SMILES N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of EHMT2 (unknown origin)-mediated dimethylation of a biotinylated histone H3 peptide at lysine-9 by FRET-ba... |
ACS Med Chem Lett 5: 293-7 (2014)
Article DOI: 10.1021/ml4002503
BindingDB Entry DOI: 10.7270/Q2N58NWS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data