

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50379274 CHEMBL2011499::US9238626, (+)-Huprine Y HCl
SMILES: CC1=C[C@@H]2C[C@H](C1)c1c(C2)nc2cc(Cl)ccc2c1N
InChI Key: InChIKey=UKCBMHDZLYYTMI-MNOVXSKESA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50379274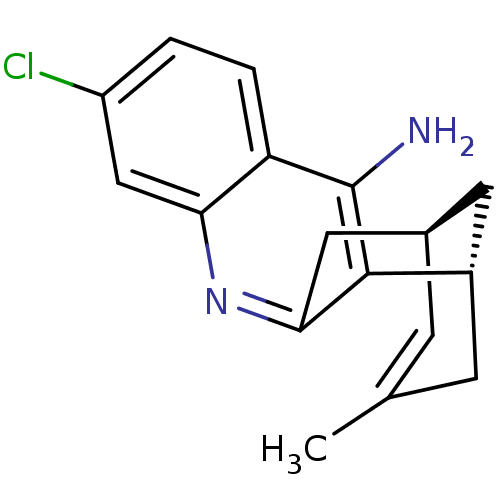 (CHEMBL2011499 | US9238626, (+)-Huprine Y HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13.6 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM50379274 (CHEMBL2011499 | US9238626, (+)-Huprine Y HCl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description BChE inhibitory activity determinations were carried out similarly by the method of Ellman et al., using 0.02 unit/mL of human serum BChE and 300 u... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50379274 (CHEMBL2011499 | US9238626, (+)-Huprine Y HCl) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description β-Secretase (BACE-1, Sigma) inhibition studies were performed by employing a peptide mimicking APP sequence as substrate (methoxycoumarin-Ser-... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50379274 (CHEMBL2011499 | US9238626, (+)-Huprine Y HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50379274 (CHEMBL2011499 | US9238626, (+)-Huprine Y HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 321 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM50379274 (CHEMBL2011499 | US9238626, (+)-Huprine Y HCl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50379274 (CHEMBL2011499 | US9238626, (+)-Huprine Y HCl) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using methoxycoumarin-Ser-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys-dinitrophenyl as substrate preincubated for 1 hr ... | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50379274 (CHEMBL2011499 | US9238626, (+)-Huprine Y HCl) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 373 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||