

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50387943 CHEMBL2058924::US9283222, 510
SMILES: Fc1ccc(Cc2n[nH]c(=O)c3CCCNc23)cc1C(=O)N1CCN(CC1)c1ccncc1
InChI Key: InChIKey=BDPJKAORASQKHG-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50387943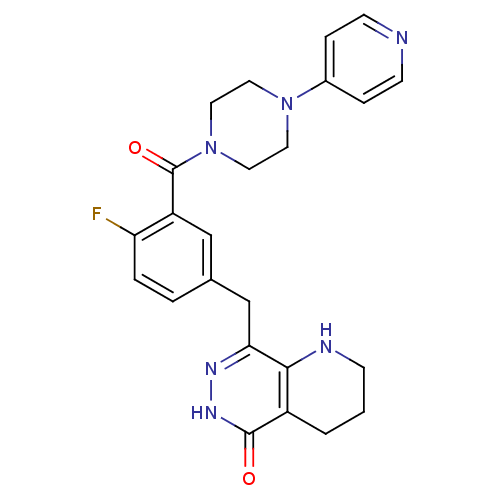 (CHEMBL2058924 | US9283222, 510) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
AbbVie Inc. US Patent | Assay Description PARP1 assay was conducted in PARP assay buffer containing 50 mM Tris pH 8.0, 1 mM DTT, 4 mM MgCl2. PARP reactions contained 1.5 uM [3H]-NAD+ (1.6 uCi... | US Patent US9283222 (2016) BindingDB Entry DOI: 10.7270/Q2BZ64XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50387943 (CHEMBL2058924 | US9283222, 510) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting | Bioorg Med Chem 20: 4635-45 (2012) Article DOI: 10.1016/j.bmc.2012.06.021 BindingDB Entry DOI: 10.7270/Q2PN96P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50387943 (CHEMBL2058924 | US9283222, 510) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 in H202-stimulated human C41 cells incubated for 30 mins prior to H2O2-treatment measured after 10 mins by FITC-based immunostain... | Bioorg Med Chem 20: 4635-45 (2012) Article DOI: 10.1016/j.bmc.2012.06.021 BindingDB Entry DOI: 10.7270/Q2PN96P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||