

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50388737 CHEMBL2058860
SMILES: CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(=O)NCC(C)c1ccccc1
InChI Key: InChIKey=SESMVBDEQODYAX-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin II receptor (Homo sapiens (Human)) | BDBM50388737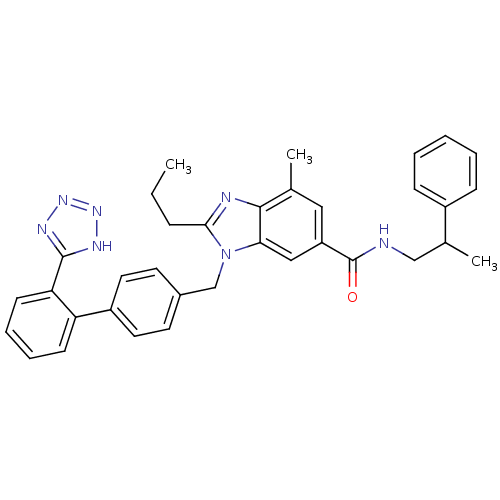 (CHEMBL2058860) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from angiotensin AT1 receptor after 180 mins by gamma counting | Bioorg Med Chem 20: 4208-16 (2012) Article DOI: 10.1016/j.bmc.2012.05.056 BindingDB Entry DOI: 10.7270/Q2GT5P7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II receptor (Homo sapiens (Human)) | BDBM50388737 (CHEMBL2058860) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from human AT1 receptor expressed in CHO-K1 cell membranes after 180 mins | Eur J Med Chem 103: 473-87 (2015) Article DOI: 10.1016/j.ejmech.2015.09.010 BindingDB Entry DOI: 10.7270/Q21J9DS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II type 1a (AT-1a) receptor (RABBIT) | BDBM50388737 (CHEMBL2058860) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment Curated by ChEMBL | Assay Description Antagonist activity at AT1 receptor in Japanese white rabbit thoracic aorta assessed as inhibition of KCl-induced contraction incubated 30 mins post ... | Bioorg Med Chem 20: 4208-16 (2012) Article DOI: 10.1016/j.bmc.2012.05.056 BindingDB Entry DOI: 10.7270/Q2GT5P7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||