

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50391839 CHEMBL2147034::US9133160, 2
SMILES: Cc1ccncc1-c1cccc2n(cnc12)-c1ccccn1
InChI Key: InChIKey=WDKLQTCTEXQEBO-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 17A1 (Homo sapiens (Human)) | BDBM50391839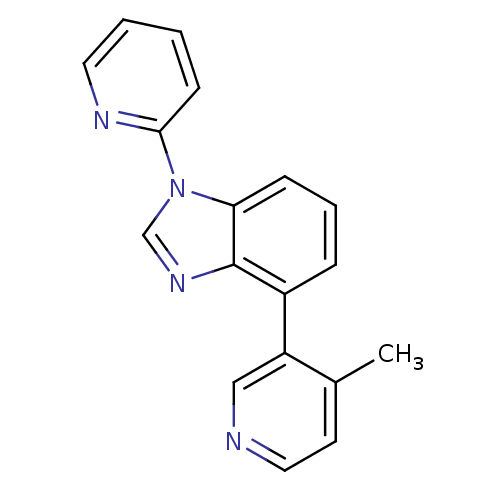 (CHEMBL2147034 | US9133160, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Bristol-Meyers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well optiplates. The final assay volume was 15 ul prepared from 7.5 ul additions of microsomes (prepared as... | US Patent US9133160 (2015) BindingDB Entry DOI: 10.7270/Q2KD1WQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 17A1 (Homo sapiens (Human)) | BDBM50391839 (CHEMBL2147034 | US9133160, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CYP17 transfected in human HEK2 cells using [3H]pregnenolone as substrate by SPA assay | ACS Med Chem Lett 3: 614-615 (2012) Article DOI: 10.1021/ml300157r BindingDB Entry DOI: 10.7270/Q2V125W6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50391839 (CHEMBL2147034 | US9133160, 2) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | ACS Med Chem Lett 7: 40-5 (2016) BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 17A1 (Homo sapiens (Human)) | BDBM50391839 (CHEMBL2147034 | US9133160, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... | ACS Med Chem Lett 7: 40-5 (2016) BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||