

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50392999 CHEMBL2152545
SMILES: CCCCCCCNC(=O)Oc1ccc2N(C)C3N(CCc4ccccc34)Cc2c1
InChI Key: InChIKey=NRSCUZWEMZYPRV-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Butyrylcholinesterase (BuChE) (Equus caballus (Horse)) | BDBM50392999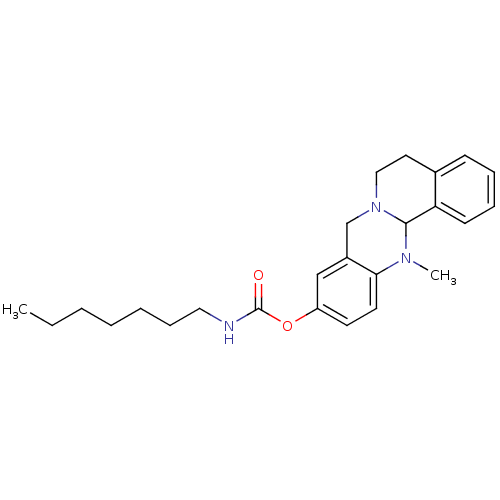 (CHEMBL2152545) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Time dependent inhibition of equine serum BChE assessed as stability constants of inhibitor-enzyme complex using acetylthiocholine as substrate after... | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Butyrylcholinesterase (BuChE) (Equus caballus (Horse)) | BDBM50392999 (CHEMBL2152545) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine as substrate after 2 mins | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases; ACHE & BCHE (Homo sapiens (Human)) | BDBM50392999 (CHEMBL2152545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University W£rzburg Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 30 mins followed by substrate addition and measured afte... | J Med Chem 62: 9116-9140 (2019) Article DOI: 10.1021/acs.jmedchem.9b01012 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50392999 (CHEMBL2152545) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University W£rzburg Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE at 10 uM using acetylthiocholine iodide as substrate preincubated for 30 mins followed by substrate addition and... | J Med Chem 62: 9116-9140 (2019) Article DOI: 10.1021/acs.jmedchem.9b01012 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50392999 (CHEMBL2152545) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University W£rzburg Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE at 10 uM using acetylthiocholine iodide as substrate preincubated for 30 mins followed by substrate addition and... | J Med Chem 62: 9116-9140 (2019) Article DOI: 10.1021/acs.jmedchem.9b01012 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Butyrylcholinesterase (BuChE) (Equus caballus (Horse)) | BDBM50392999 (CHEMBL2152545) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine as substrate after 2 mins | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases; ACHE & BCHE (Homo sapiens (Human)) | BDBM50392999 (CHEMBL2152545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University W£rzburg Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 30 mins followed by substrate addition and measured afte... | J Med Chem 62: 9116-9140 (2019) Article DOI: 10.1021/acs.jmedchem.9b01012 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||