

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50396228 CHEMBL2172183::US8569318, 1.2(3)
SMILES: CSc1nn2c3CCN(C)Cc3cnc2c1S(=O)(=O)c1ccccc1
InChI Key: InChIKey=SLKVTBZWWJECHL-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50396228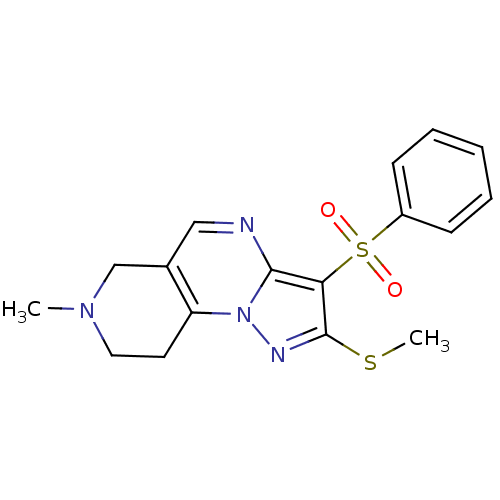 (CHEMBL2172183 | US8569318, 1.2(3)) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]lysergic acid diethylamide from human recombinant 5HT6 receptor expressed in HeLa cells after 120 mins | Bioorg Med Chem Lett 22: 4273-80 (2012) Article DOI: 10.1016/j.bmcl.2012.05.036 BindingDB Entry DOI: 10.7270/Q2VH5PZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50396228 (CHEMBL2172183 | US8569318, 1.2(3)) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human recombinant 5HT6 receptor expressed in HE293 cells assessed as inhibition of serotonin-induced cAMP accumulation after 2... | Bioorg Med Chem Lett 22: 4273-80 (2012) Article DOI: 10.1016/j.bmcl.2012.05.036 BindingDB Entry DOI: 10.7270/Q2VH5PZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50396228 (CHEMBL2172183 | US8569318, 1.2(3)) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | 35.7 | n/a | 76.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Activity test of serotonin 5-HT6 receptor antagonists of the general formulas 1, 2 in the setting of competitive binding to serotonin 5-HT6 receptors... | US Patent US8569318 (2013) BindingDB Entry DOI: 10.7270/Q2JH3JT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||