

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50396426 CHEMBL2170399
SMILES: [#6]-[#6]-[#6]-[#6]-[#7](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]
InChI Key: InChIKey=WUEUJMYZZQSJPB-BBACVFHCSA-N
Data: 1 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GPR103 (Homo sapiens (Human)) | BDBM50396426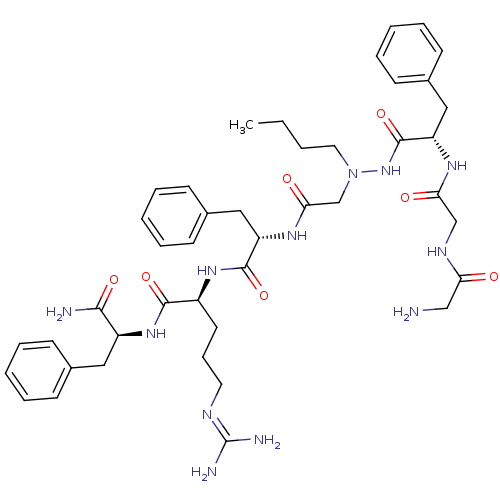 (CHEMBL2170399) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.98E+4 | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Agonist activity at human GPR103 expressed in CHO cells co-expressing Galpha16 assessed as increase in intracellular calcium level measured for 2 min... | J Med Chem 55: 7516-24 (2012) Article DOI: 10.1021/jm300507d BindingDB Entry DOI: 10.7270/Q2K35VSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||