

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(C)Oc1nc(nc2CCN(Cc12)S(=O)(=O)c1cccnc1)-c1ccc(Cl)nc1
InChI Key: InChIKey=GGRRBUUAIYPJKH-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50398012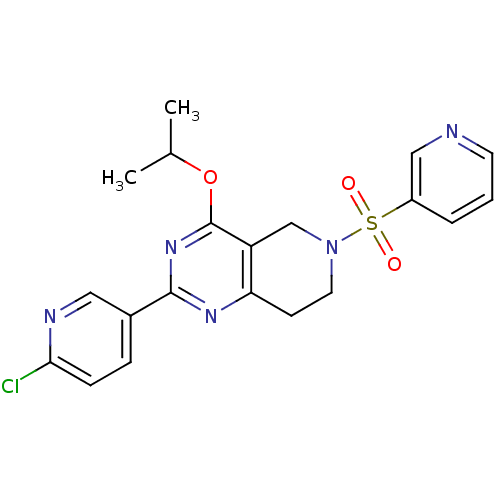 (CHEMBL2180422 | US8492392, 1-17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PDE10A by fluorescence polarization assay | J Med Chem 55: 7299-331 (2012) Article DOI: 10.1021/jm3004976 BindingDB Entry DOI: 10.7270/Q2C24XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50398012 (CHEMBL2180422 | US8492392, 1-17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 0.630 | -12.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||