

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50407372 CHEMBL44220
SMILES: CNC(=S)NCCCCCc1cnc[nH]1
InChI Key: InChIKey=BHXVXFKQYAHZKJ-UHFFFAOYSA-N
Data: 2 Kd
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HRH3 (GUINEA PIG) | BDBM50407372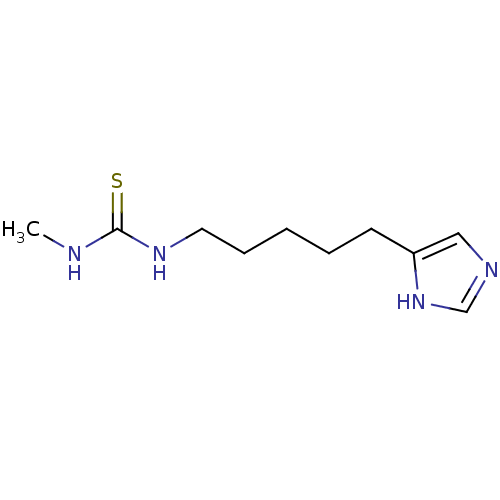 (CHEMBL44220) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description In vitro inhibitory effect of histamine H3 antagonist on the electrically evoked contractile response of isolated guinea pig jejunum segments. | J Med Chem 38: 2244-50 (1995) BindingDB Entry DOI: 10.7270/Q2RF5W7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HRH3 (GUINEA PIG) | BDBM50407372 (CHEMBL44220) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex | J Med Chem 44: 1666-74 (2001) BindingDB Entry DOI: 10.7270/Q2WH2QP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||