

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50419205 CHEMBL1836172
SMILES: CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CCN(CCc2c1)C(CO)CO
InChI Key: InChIKey=MSPRXNQLTFALLV-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50419205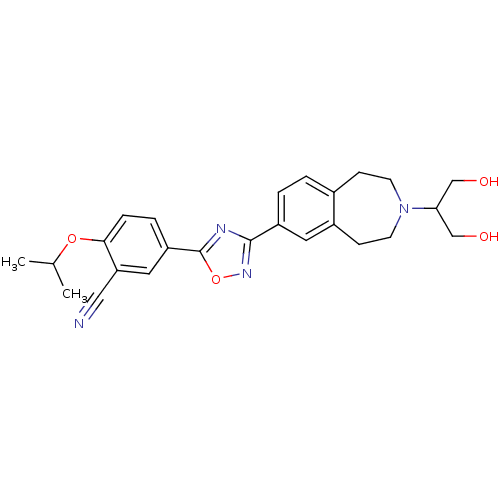 (CHEMBL1836172) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human S1P1 receptor expressed in CHO-K1 EDG1 cells expressing beta-arrestin 2 after 105 mins by chemi-luminiscence assay | J Med Chem 54: 6724-33 (2011) Article DOI: 10.1021/jm200609t BindingDB Entry DOI: 10.7270/Q27P8ZSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50419205 (CHEMBL1836172) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human ERG | J Med Chem 54: 6724-33 (2011) Article DOI: 10.1021/jm200609t BindingDB Entry DOI: 10.7270/Q27P8ZSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50419205 (CHEMBL1836172) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in RBL cells assessed as [35S]GTPgammaS binding after 3 hrs by radiometric assay | J Med Chem 54: 6724-33 (2011) Article DOI: 10.1021/jm200609t BindingDB Entry DOI: 10.7270/Q27P8ZSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||