 Found 6 hits for monomerid = 50422364
Found 6 hits for monomerid = 50422364 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase HD2
(Zea mays) | BDBM50422364
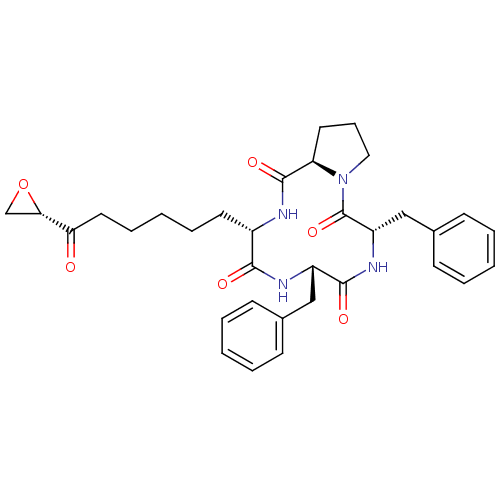
(TRAPOXIN B)Show SMILES O=C(CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H]1CO1 Show InChI InChI=1S/C33H40N4O6/c38-28(29-21-43-29)17-9-3-8-15-24-30(39)35-25(19-22-11-4-1-5-12-22)31(40)36-26(20-23-13-6-2-7-14-23)33(42)37-18-10-16-27(37)32(41)34-24/h1-2,4-7,11-14,24-27,29H,3,8-10,15-21H2,(H,34,41)(H,35,39)(H,36,40)/t24-,25-,26-,27+,29-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibitory concentration against histone deacetylase activity |
J Med Chem 44: 2069-72 (2001)
BindingDB Entry DOI: 10.7270/Q2959GT3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Mus musculus (Mouse)) | BDBM50422364

(TRAPOXIN B)Show SMILES O=C(CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H]1CO1 Show InChI InChI=1S/C33H40N4O6/c38-28(29-21-43-29)17-9-3-8-15-24-30(39)35-25(19-22-11-4-1-5-12-22)31(40)36-26(20-23-13-6-2-7-14-23)33(42)37-18-10-16-27(37)32(41)34-24/h1-2,4-7,11-14,24-27,29H,3,8-10,15-21H2,(H,34,41)(H,35,39)(H,36,40)/t24-,25-,26-,27+,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibitory concentration gainst Histone deacetylase 1 derived from NIH3T3 cells |
J Med Chem 47: 2984-94 (2004)
Article DOI: 10.1021/jm030222i
BindingDB Entry DOI: 10.7270/Q2G44PR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50422364

(TRAPOXIN B)Show SMILES O=C(CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H]1CO1 Show InChI InChI=1S/C33H40N4O6/c38-28(29-21-43-29)17-9-3-8-15-24-30(39)35-25(19-22-11-4-1-5-12-22)31(40)36-26(20-23-13-6-2-7-14-23)33(42)37-18-10-16-27(37)32(41)34-24/h1-2,4-7,11-14,24-27,29H,3,8-10,15-21H2,(H,34,41)(H,35,39)(H,36,40)/t24-,25-,26-,27+,29-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit human Histone deacetylase (HDAC) enzyme by 50% |
J Med Chem 45: 753-7 (2002)
Article DOI: 10.1021/jm015568c
BindingDB Entry DOI: 10.7270/Q2T156D5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50422364

(TRAPOXIN B)Show SMILES O=C(CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H]1CO1 Show InChI InChI=1S/C33H40N4O6/c38-28(29-21-43-29)17-9-3-8-15-24-30(39)35-25(19-22-11-4-1-5-12-22)31(40)36-26(20-23-13-6-2-7-14-23)33(42)37-18-10-16-27(37)32(41)34-24/h1-2,4-7,11-14,24-27,29H,3,8-10,15-21H2,(H,34,41)(H,35,39)(H,36,40)/t24-,25-,26-,27+,29-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50422364

(TRAPOXIN B)Show SMILES O=C(CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H]1CO1 Show InChI InChI=1S/C33H40N4O6/c38-28(29-21-43-29)17-9-3-8-15-24-30(39)35-25(19-22-11-4-1-5-12-22)31(40)36-26(20-23-13-6-2-7-14-23)33(42)37-18-10-16-27(37)32(41)34-24/h1-2,4-7,11-14,24-27,29H,3,8-10,15-21H2,(H,34,41)(H,35,39)(H,36,40)/t24-,25-,26-,27+,29-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the partially purified HDAC enzyme by 50% obtained from H1299 cell lysate |
J Med Chem 45: 753-7 (2002)
Article DOI: 10.1021/jm015568c
BindingDB Entry DOI: 10.7270/Q2T156D5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase HD2
(Zea mays) | BDBM50422364

(TRAPOXIN B)Show SMILES O=C(CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H]1CO1 Show InChI InChI=1S/C33H40N4O6/c38-28(29-21-43-29)17-9-3-8-15-24-30(39)35-25(19-22-11-4-1-5-12-22)31(40)36-26(20-23-13-6-2-7-14-23)33(42)37-18-10-16-27(37)32(41)34-24/h1-2,4-7,11-14,24-27,29H,3,8-10,15-21H2,(H,34,41)(H,35,39)(H,36,40)/t24-,25-,26-,27+,29-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza"
Curated by ChEMBL
| Assay Description
Inhibitory concentration against maize histone deacetylase 2 |
J Med Chem 48: 3344-53 (2005)
Article DOI: 10.1021/jm049002a
BindingDB Entry DOI: 10.7270/Q2222VKN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data