

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50425813 CHEMBL2316897
SMILES: OC(=O)c1cc2c(\C=C/c3ccc(OC(F)(F)F)cc3)c(oc2cc1O)-c1ccccc1
InChI Key: InChIKey=FCEHHFXUHSFLBJ-FLIBITNWSA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dual specificity protein phosphatase (VHR) (Homo sapiens (Human)) | BDBM50425813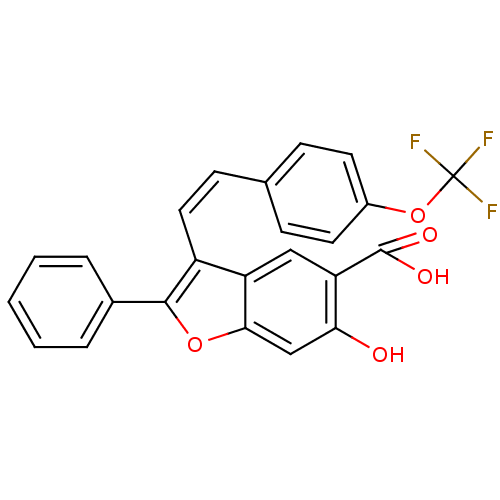 (CHEMBL2316897) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of VHR (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte common antigen (Homo sapiens (Human)) | BDBM50425813 (CHEMBL2316897) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of CD45 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase MEG2 (PTP-Meg2) (Homo sapiens (Human)) | BDBM50425813 (CHEMBL2316897) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50425813 (CHEMBL2316897) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50425813 (CHEMBL2316897) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1C (Homo sapiens (Human)) | BDBM50425813 (CHEMBL2316897) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP1 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50425813 (CHEMBL2316897) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of TCPTP (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic cell protein-tyrosine phosphatase 70Z-PEP (Homo sapiens (Human)) | BDBM50425813 (CHEMBL2316897) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of LYP (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||