

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Cc1cc2c(cc1-n1c(nc3cc(ccc13)C(O)=O)C(F)(F)F)C(C)(C)CCC2(C)C
InChI Key: InChIKey=GBMUQLAGEWYKTJ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50429866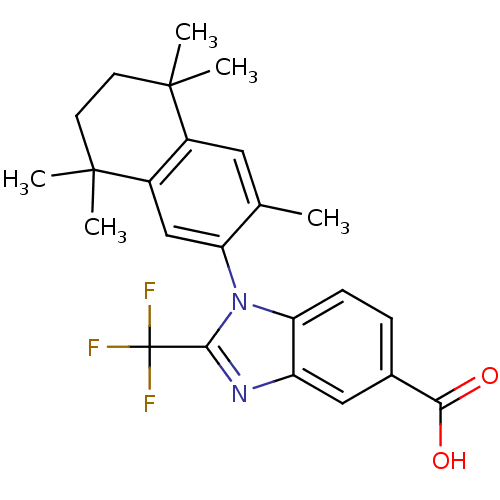 (CHEMBL2332887) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Agonist activity at human RXRalpha expressed in African green monkey COS1 cells harboring CRBP2-tk-luc reporter incubated for 18 hrs by steady-glo lu... | Bioorg Med Chem Lett 29: 1891-1894 (2019) Article DOI: 10.1016/j.bmcl.2019.05.050 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50429866 (CHEMBL2332887) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Partial agonist activity at RXRalpha (unknown origin) expressed in COS1 cells after 18 hrs by luciferase reporter gene assay | J Med Chem 56: 1865-77 (2013) Article DOI: 10.1021/jm400033f BindingDB Entry DOI: 10.7270/Q2348MR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||