

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50431242 CHEMBL2333026
SMILES: C[C@@H](NC(=O)c1ccncc1)C(=O)N1CCC[C@H]1B(O)O
InChI Key: InChIKey=DRBWRJPFNOBNIO-KOLCDFICSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fibroblast activation protein alpha (Homo sapiens (Human)) | BDBM50431242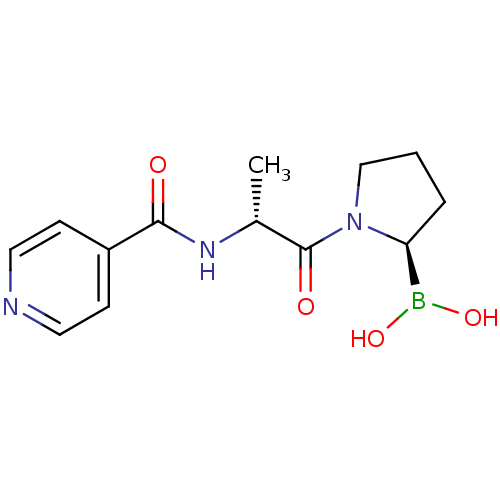 (CHEMBL2333026) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Competitive inhibition of human FAP using Suc-GP-AMC as substrate by Lineweaver-Burk plot analysis | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50431242 (CHEMBL2333026) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Competitive inhibition of human PREP using Suc-GP-AMC as substrate by Lineweaver-Burk plot analysis | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50431242 (CHEMBL2333026) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of recombinant human PREP purified from Escherichia coli using Z-Gly-Pro-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431242 (CHEMBL2333026) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of DPP4 purified from human seminal plasma using Gly-Pro-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50431242 (CHEMBL2333026) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Inhibition of human PREP using Z-Gly-Pro-AMC as substrate preincubated for 10 mins prior to substrate addition by fluorescence assay | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast activation protein alpha (Homo sapiens (Human)) | BDBM50431242 (CHEMBL2333026) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Inhibition of human FAP using Z-Gly-Pro-AMC as substrate preincubated for 10 mins prior to substrate addition by fluorescence assay | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50431242 (CHEMBL2333026) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of POP (unknown origin) | J Med Chem 62: 7874-7884 (2019) Article DOI: 10.1021/acs.jmedchem.9b00642 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase VIII (Homo sapiens (Human)) | BDBM50431242 (CHEMBL2333026) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Inhibition of human DPP8 using H-Gly-Pro-AMC as substrate preincubated for 10 mins prior to substrate addition by fluorescence assay | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431242 (CHEMBL2333026) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate preincubated for 10 mins prior to substrate addition by fluorescence assay | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-protein thioesterase 1/2 (Rattus norvegicus) | BDBM50431242 (CHEMBL2333026) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >36 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of FAP (unknown origin) | J Med Chem 62: 7874-7884 (2019) Article DOI: 10.1021/acs.jmedchem.9b00642 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast activation protein (FAP) (Mus musculus (Mouse)) | BDBM50431242 (CHEMBL2333026) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of recombinant mouse FAP purified from HEK293 cell supernatant using Ala-Pro-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (DPP II) (Homo sapiens (Human)) | BDBM50431242 (CHEMBL2333026) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of DPP2 purified from human seminal plasma using Lys-Ala-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50431242 (CHEMBL2333026) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Inhibition of human DPP9 using H-Gly-Pro-AMC as substrate preincubated for 10 mins prior to substrate addition by fluorescence assay | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||