

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50434070 CHEMBL2381340
SMILES: CCOc1cc(ccc1Nc1ncc2N(C)C(=O)c3ccccc3N(C3CCCC3)c2n1)C(=O)N1CCC(CC1)N1CCN(C)CC1
InChI Key: InChIKey=XVBGRTMNFNMINE-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extracellular signal-regulated kinase 5 (ERK5) (Homo sapiens (Human)) | BDBM50434070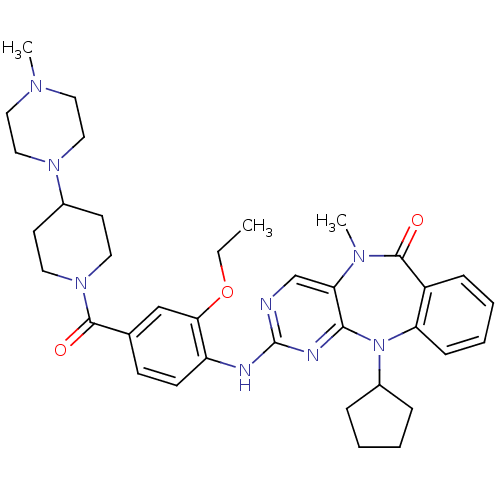 (CHEMBL2381340) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length human ERK5 transfected in HEK293 cells assessed as inhibition of AP1 transcriptional activity after 24 hrs by du... | J Med Chem 56: 4413-21 (2013) Article DOI: 10.1021/jm4000837 BindingDB Entry DOI: 10.7270/Q2RF5WDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50434070 (CHEMBL2381340) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 171 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 catalytic domain (970 to 2527 residues) expressed in baculovirus expression system using LRRKtide as... | J Med Chem 63: 7817-7826 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase DCLK1 (Homo sapiens (Human)) | BDBM50434070 (CHEMBL2381340) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged DCLK1 (G351 to H689 residues) expressed in Escherichia coli BL21 DE3 using 5-FAM-KKLRRTLSVA-CO... | J Med Chem 63: 7817-7826 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||