

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50439795 CHEMBL2419702::US9181266, 34
SMILES: CCCN(Cc1nc2CCOCc2c(=O)[nH]1)C(=O)CN1CCC(CC1)C(=O)c1ccc(F)cc1
InChI Key: InChIKey=CCVBOAMGJQRBBT-UHFFFAOYSA-N
Data: 7 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50439795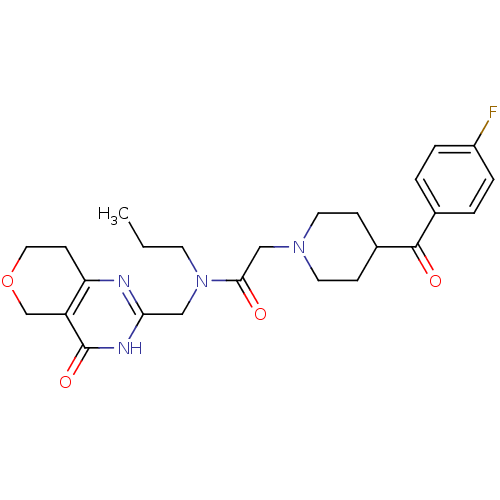 (CHEMBL2419702 | US9181266, 34) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG US Patent | Assay Description The autoparsylation activity of the TNKS 1/2 or PARP1/2 enzymes was measured by the liquid chromatography-mass spectrometry (LC/MS) detection of nico... | US Patent US9181266 (2015) BindingDB Entry DOI: 10.7270/Q20P0XTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50439795 (CHEMBL2419702 | US9181266, 34) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG US Patent | Assay Description The autoparsylation activity of the TNKS 1/2 or PARP1/2 enzymes was measured by the liquid chromatography-mass spectrometry (LC/MS) detection of nico... | US Patent US9181266 (2015) BindingDB Entry DOI: 10.7270/Q20P0XTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50439795 (CHEMBL2419702 | US9181266, 34) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG US Patent | Assay Description The autoparsylation activity of the TNKS 1/2 or PARP1/2 enzymes was measured by the liquid chromatography-mass spectrometry (LC/MS) detection of nico... | US Patent US9181266 (2015) BindingDB Entry DOI: 10.7270/Q20P0XTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50439795 (CHEMBL2419702 | US9181266, 34) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PARP1 (unknown origin) assessed as nicotinamide concentration by LC-MS analysis | J Med Chem 56: 6495-511 (2013) Article DOI: 10.1021/jm400807n BindingDB Entry DOI: 10.7270/Q24Q7WFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Wnt-3a (Homo sapiens (Human)) | BDBM50439795 (CHEMBL2419702 | US9181266, 34) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of WNT3A signaling in HEK293 cells by luciferase reporter gene assay in presence of forskolin | J Med Chem 56: 6495-511 (2013) Article DOI: 10.1021/jm400807n BindingDB Entry DOI: 10.7270/Q24Q7WFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50439795 (CHEMBL2419702 | US9181266, 34) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PARP2 (unknown origin) assessed as nicotinamide concentration by LC-MS analysis | J Med Chem 56: 6495-511 (2013) Article DOI: 10.1021/jm400807n BindingDB Entry DOI: 10.7270/Q24Q7WFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50439795 (CHEMBL2419702 | US9181266, 34) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG US Patent | Assay Description The autoparsylation activity of the TNKS 1/2 or PARP1/2 enzymes was measured by the liquid chromatography-mass spectrometry (LC/MS) detection of nico... | US Patent US9181266 (2015) BindingDB Entry DOI: 10.7270/Q20P0XTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||