

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50441566 CHEMBL2436977::US10485800, Example 179
SMILES: CN1CCN(Cc2ccc(cc2)-c2cnc3[nH]cc(-c4cnc5[nH]ccc5c4)c3c2)CC1
InChI Key: InChIKey=GOTKZROZHBWZJH-UHFFFAOYSA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibitor of nuclear factor kappa-B kinase subunit alpha (Homo sapiens (Human)) | BDBM50441566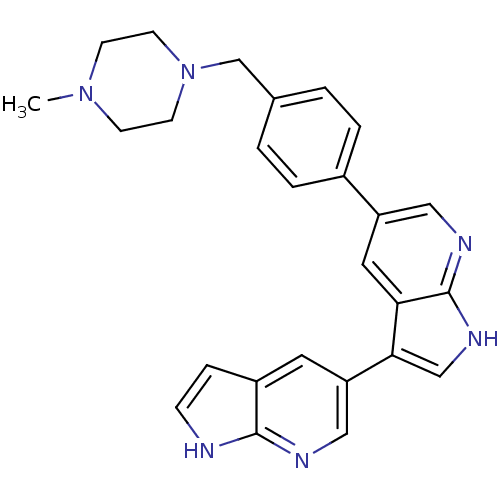 (CHEMBL2436977 | US10485800, Example 179) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Califia Bio Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human IKKa by high throughput ATP-[33P] radiolabeled assay | J Med Chem 56: 8032-48 (2013) Article DOI: 10.1021/jm401094t BindingDB Entry DOI: 10.7270/Q2P270KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50441566 (CHEMBL2436977 | US10485800, Example 179) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
Califia Bio Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human IKKb by high throughput ATP-[33P] radiolabeled assay | J Med Chem 56: 8032-48 (2013) Article DOI: 10.1021/jm401094t BindingDB Entry DOI: 10.7270/Q2P270KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50441566 (CHEMBL2436977 | US10485800, Example 179) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Califia Bio Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human FLT3 by high throughput ATP-[33P] radiolabeled assay | J Med Chem 56: 8032-48 (2013) Article DOI: 10.1021/jm401094t BindingDB Entry DOI: 10.7270/Q2P270KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 11 (Homo sapiens (Human)) | BDBM50441566 (CHEMBL2436977 | US10485800, Example 179) | PDB NCI pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Rochester; Board of Regents of the University of Nebraska US Patent | Assay Description 200 ng (130 nM) MLK3 (Dundee, DU8313) was incubated with 1 μM inactive MKK7b (Dundee, DU703) in the presence of 2 μM cold ATP (Km) and 0.5 ... | US Patent US10485800 (2019) BindingDB Entry DOI: 10.7270/Q22B91DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50441566 (CHEMBL2436977 | US10485800, Example 179) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Califia Bio Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human IR by high throughput ATP-[33P] radiolabeled assay | J Med Chem 56: 8032-48 (2013) Article DOI: 10.1021/jm401094t BindingDB Entry DOI: 10.7270/Q2P270KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50441566 (CHEMBL2436977 | US10485800, Example 179) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Califia Bio Inc. Curated by ChEMBL | Assay Description Inhibition of FLT3-L-stimulated wild type full length human FLT3 autophosphorylation expressed in MEF preincubated for 90 mins followed by FLT3-L sti... | J Med Chem 56: 8032-48 (2013) Article DOI: 10.1021/jm401094t BindingDB Entry DOI: 10.7270/Q2P270KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 11 (Homo sapiens (Human)) | BDBM50441566 (CHEMBL2436977 | US10485800, Example 179) | PDB NCI pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Rochester; Board of Regents of the University of Nebraska US Patent | Assay Description 200 ng (130 nM) MLK3 (Dundee, DU8313) was incubated with 1 μM inactive MKK7b (Dundee, DU703) in the presence of 2 μM cold ATP (Km) and 0.5 ... | US Patent US10485800 (2019) BindingDB Entry DOI: 10.7270/Q22B91DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 11 (Homo sapiens (Human)) | BDBM50441566 (CHEMBL2436977 | US10485800, Example 179) | PDB NCI pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Califia Bio Inc. Curated by ChEMBL | Assay Description Inhibition of MLK3 (unknown origin) after 20 mins by scintillation counting analysis in presence of [33P]-ATP | J Med Chem 56: 8032-48 (2013) Article DOI: 10.1021/jm401094t BindingDB Entry DOI: 10.7270/Q2P270KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||