 Found 5 hits for monomerid = 50441626
Found 5 hits for monomerid = 50441626 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50441626
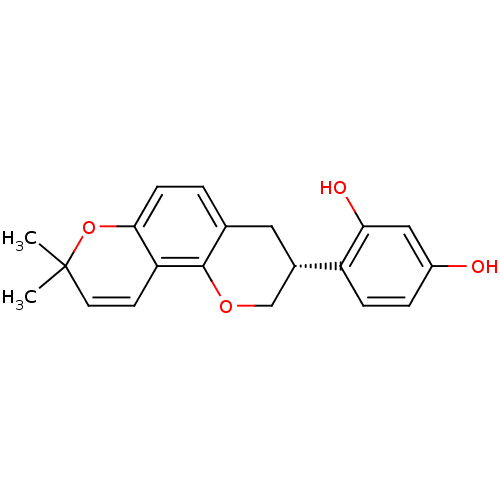
(GLABRIDIN | US9783551, Compound 1)Show SMILES CC1(C)Oc2ccc3C[C@@H](COc3c2C=C1)c1ccc(O)cc1O |r,c:16| Show InChI InChI=1S/C20H20O4/c1-20(2)8-7-16-18(24-20)6-3-12-9-13(11-23-19(12)16)15-5-4-14(21)10-17(15)22/h3-8,10,13,21-22H,9,11H2,1-2H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 CYP3A4 measured by 7BFC O-debenzylation |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50441626

(GLABRIDIN | US9783551, Compound 1)Show SMILES CC1(C)Oc2ccc3C[C@@H](COc3c2C=C1)c1ccc(O)cc1O |r,c:16| Show InChI InChI=1S/C20H20O4/c1-20(2)8-7-16-18(24-20)6-3-12-9-13(11-23-19(12)16)15-5-4-14(21)10-17(15)22/h3-8,10,13,21-22H,9,11H2,1-2H3/t13-/m0/s1 | KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase (unknown origin) using L-tyrosine as substrate preincubated for 5 mins followed by substrate addition |
J Nat Prod 80: 334-346 (2017)
Article DOI: 10.1021/acs.jnatprod.6b00783
BindingDB Entry DOI: 10.7270/Q2XD146V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50441626

(GLABRIDIN | US9783551, Compound 1)Show SMILES CC1(C)Oc2ccc3C[C@@H](COc3c2C=C1)c1ccc(O)cc1O |r,c:16| Show InChI InChI=1S/C20H20O4/c1-20(2)8-7-16-18(24-20)6-3-12-9-13(11-23-19(12)16)15-5-4-14(21)10-17(15)22/h3-8,10,13,21-22H,9,11H2,1-2H3/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaceum Inc.
US Patent
| Assay Description
The degree of inhibition activity for PTP1B was investigated by using 2 mM p-nitrophenyl phosphate (p-NPP) as a substrate to measure the dephosphoryl... |
US Patent US9783551 (2017)
BindingDB Entry DOI: 10.7270/Q2CV4KWV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50441626

(GLABRIDIN | US9783551, Compound 1)Show SMILES CC1(C)Oc2ccc3C[C@@H](COc3c2C=C1)c1ccc(O)cc1O |r,c:16| Show InChI InChI=1S/C20H20O4/c1-20(2)8-7-16-18(24-20)6-3-12-9-13(11-23-19(12)16)15-5-4-14(21)10-17(15)22/h3-8,10,13,21-22H,9,11H2,1-2H3/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using p-nitrophenyl phosphate as substrate |
Bioorg Med Chem Lett 23: 5836-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.102
BindingDB Entry DOI: 10.7270/Q28W3FQ0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50441626

(GLABRIDIN | US9783551, Compound 1)Show SMILES CC1(C)Oc2ccc3C[C@@H](COc3c2C=C1)c1ccc(O)cc1O |r,c:16| Show InChI InChI=1S/C20H20O4/c1-20(2)8-7-16-18(24-20)6-3-12-9-13(11-23-19(12)16)15-5-4-14(21)10-17(15)22/h3-8,10,13,21-22H,9,11H2,1-2H3/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaceum Inc.
US Patent
| Assay Description
The degree of inhibition activity for PTP1B was investigated by using 2 mM p-nitrophenyl phosphate (p-NPP) as a substrate to measure the dephosphoryl... |
US Patent US9783551 (2017)
BindingDB Entry DOI: 10.7270/Q2CV4KWV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data